Abstract
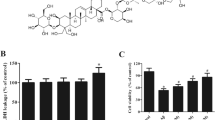
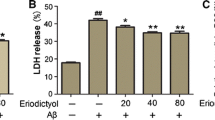
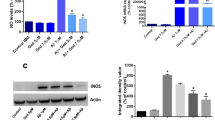
Alzheimer’s disease (AD) is a serious neuropathologic disease characterized by aggregation of amyloid-β (Aβ) peptide. Aβ-mediated oxidative stress and neuroinflammation play crucial role in the development of AD. Engeletin is a flavononol glycoside that possesses anti-inflammatory effect. However, the effects of engeletin on AD have not been investigated. In the present study, we investigated the role of engeletin in AD using an in vitro AD model. Murine microglia BV-2 cells were stimulated with Aβ1–42 (5 μM) for 24 h to induce oxidative stress and inflammation. Our results showed that treatment with engeletin suppressed Aβ1–42-induced viability reduction and lactate dehydrogenase (LDH) release in BV-2 cells. Engeletin attenuated Aβ1–42-induced oxidative stress in BV-2 cells, as proved by decreased production of reactive oxygen species (ROS) and malonaldehyde (MDA) and increased glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) activities. Aβ1–42-induced nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) expression were inhibited by engeletin treatment. Besides, engeletin inhibited Aβ1–42-induced production and mRNA levels of tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6). Engeletin enhanced Aβ1–42-induced activation of Kelch-like ECH-associated protein 1 (Keap1)/nuclear transcription factor E2-related factor 2 (Nrf2) signaling pathway in BV-2 cells. Inhibition of Keap1/Nrf2 signaling pathway reversed the inhibitory effects of engeletin on Aβ1–42-induced oxidative stress and inflammation in BV-2 cells. Taken together, engeletin attenuated Aβ1–42-induced oxidative stress and inflammation in BV-2 cells via regulating the of Keap1/Nrf2 pathway. These findings indicated that engeletin might be served as a therapeutic agent for the treatment of AD.









Similar content being viewed by others
References
Calderon-Garciduenas, A.L., and C. Duyckaerts. 2017. Alzheimer disease. Handbook of Clinical Neurology 145: 325–337.
Gouras, G.K., T.T. Olsson, and O. Hansson. 2015. β-Amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics 12: 3–11.
Reiss, A.B., H.A. Arain, M.M. Stecker, N.M. Siegart, and L.J. Kasselman. 2018. Amyloid toxicity in Alzheimer’s disease. Reviews in the Neurosciences 29: 613–627.
Nazem, A., R. Sankowski, M. Bacher, and Y. Al-Abed. 2015. Rodent models of neuroinflammation for Alzheimer’s disease. Journal of Neuroinflammation 12: 74.
Minter, M.R., J.M. Taylor, and P.J. Crack. 2016. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. Journal of Neurochemistry 136: 457–474.
Viola, K.L., and W.L. Klein. 2015. Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathologica 129: 183–206.
Tian, Q., G. Wang, Y. Zhang, F. Zhang, L. Yang, Z. Liu, and Z. Shen. 2019. Engeletin inhibits lipopolysaccharide/d-galactosamine-induced liver injury in mice through activating PPAR-γ. Journal of Pharmacological Sciences 140: 218–222.
Jiang, X., L. Chen, Z. Zhang, Y. Sun, X. Wang, and J. Wei. 2018. Protective and therapeutic effects of engeletin on LPS-induced acute lung injury. Inflammation 41: 1259–1265.
Wu, H., G. Zhao, K. Jiang, C. Li, C. Qiu, and G. Deng. 2016. Engeletin alleviates lipopolysaccharide-induced endometritis in mice by inhibiting TLR4-mediated NF-κB activation. Journal of Agricultural and Food Chemistry 64: 6171–6178.
Li, Q., L. Chen, X. Liu, X. Li, Y. Cao, Y. Bai, and F. Qi. 2018. Pterostilbene inhibits amyloid-β-induced neuroinflammation in a microglia cell line by inactivating the NLRP3/caspase-1 inflammasome pathway. Journal of Cellular Biochemistry 119: 7053–7062.
Conti, A., M. Miscusi, S. Cardali, A. Germanò, H. Suzuki, S. Cuzzocrea, and F. Tomasello. 2007. Nitric oxide in the injured spinal cord: Synthases cross-talk, oxidative stress and inflammation. Brain Research Reviews 54: 205–218.
Lu, M.C., J.A. Ji, Z.Y. Jiang, and Q.D. You. 2016. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: An update. Medicinal Research Reviews 36: 924–963.
Abdel-Hafiz, L., A. Muller-Schiffmann, C. Korth, B. Fazari, O.Y. Chao, S. Nikolaus, S. Schable, A. Herring, K. Keyvani, V. Lamounier-Zepter, J.P. Huston, and M.A. de Souza Silva. 2018. Aβ dimers induce behavioral and neurochemical deficits of relevance to early Alzheimer’s disease. Neurobiology of Aging 69: 1–9.
Perez, S.E., M.A. Raghanti, P.R. Hof, L. Kramer, and E.J. Mufson. 2013. Alzheimer’s disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla). Journal of Comparative Neurology 521: 4318–4338.
Rhein, V., G. Baysang, S. Rao, F. Meier, A. Bonert, F. Muller-Spahn, and A. Eckert. 2009. Amyloid-β leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma cells. Cellular and Molecular Neurobiology 29: 1063–1071.
Swerdlow, R.H. 2012. Mitochondria and cell bioenergetics: Increasingly recognized components and a possible etiologic cause of Alzheimer’s disease. Antioxidants & Redox Signaling 16: 1434–1455.
Elkamhawy, A., J.E. Park, A.H.E. Hassan, A.N. Pae, J. Lee, B.G. Park, and E.J. Roh. 2018. Synthesis and evaluation of 2-(3-arylureido)pyridines and 2-(3-arylureido)pyrazines as potential modulators of Aβ-induced mitochondrial dysfunction in Alzheimer’s disease. European Journal of Medicinal Chemistry 144: 529–543.
Sadigh-Eteghad, S., B. Sabermarouf, A. Majdi, M. Talebi, M. Farhoudi, and J. Mahmoudi. 2015. Amyloid-β: A crucial factor in Alzheimer’s disease. Medical Principles and Practice 24: 1–10.
Schieber, M., and N.S. Chandel. 2014. ROS function in redox signaling and oxidative stress. Current Biology 24: R453–R462.
Kamat, P.K., A. Kalani, S. Rai, S. Swarnkar, S. Tota, C. Nath, and N. Tyagi. 2016. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: Understanding the therapeutics strategies. Molecular Neurobiology 53: 648–661.
Webers, A., M.T. Heneka, and P.A. Gleeson. 2019. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunology Cell Biology 98: 28–41.
Shin, S.J., S.G. Jeon, J.I. Kim, Y.O. Jeong, S. Kim, Y.H. Park, S.K. Lee, H.H. Park, S.B. Hong, S. Oh, J.Y. Hwang, H.. Kim, H.H. Park, Y. Nam, Y.Y. Lee, J.J. Kim, S.H. Park, J.S. Kim, and M. Moon. 2019. Red ginseng attenuates Aβ-induced mitochondrial dysfunction and Aβ-mediated pathology in an animal model of Alzheimer’s disease. International Journal of Molecular Sciences 20: 3030.
Ma, W., M. Wu, S. Zhou, Y. Tao, Z. Xie, and Y. Zhong. 2018. Reduced smoothened level rescues Aβ-induced memory deficits and neuronal inflammation in animal models of Alzheimer’s disease. Journal of Genetics and Genomics 45: 237–246.
Wu, X., J. Kosaraju, and K.Y. Tam. 2018. Anti-neuroinflammatory effects of SLOH in Aβ-induced BV-2 microglial cells and 3xTg-AD mice involve the inhibition of GSK-3β. Neuroscience Letters 687: 207–215.
Cui, B., S.L. Zhang, Y.T. Wang, and Y.Y. Guo. 2019. Farrerol attenuates β-amyloid-induced oxidative stress and inflammation through Nrf2/Keap1 pathway in a microglia cell line. Biomedicine & Pharmacotherapy 109: 112–119.
Shaw, P., and A. Chattopadhyay. 2020. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. Journal of Cellular Physiology 235: 3119–3130.
Kaspar, J.W., S.K. Niture, and A.K. Jaiswal. 2009. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radical Biology and Medicine 47: 1304–1309.
Kobayashi, M., and M. Yamamoto. 2005. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxidants & Redox Signaling 7: 385–394.
Motohashi, H., and M. Yamamoto. 2004. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends in Molecular Medicine 10: 549–557.
Yamazaki, H., K. Tanji, K. Wakabayashi, S. Matsuura, and K. Itoh. 2015. Role of the Keap1/Nrf2 pathway in neurodegenerative diseases. Pathology International 65: 210–219.
Bahn, G., and D.G. Jo. 2019. Therapeutic approaches to Alzheimer’s disease through modulation of NRF2. Neuromolecular Medicine 21: 1–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhixiong Huang, Hu Ji are co-first authors.
Rights and permissions
About this article
Cite this article
Huang, Z., Ji, H., Shi, J. et al. Engeletin Attenuates Aβ1–42-Induced Oxidative Stress and Neuroinflammation by Keap1/Nrf2 Pathway. Inflammation 43, 1759–1771 (2020). https://doi.org/10.1007/s10753-020-01250-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01250-9