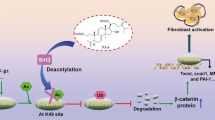
Abstract
Norcantharidin (NCTD) is a potential anti-renal interstitial fibrotic drug. However, the underlying molecular mechanism of how NCTD works remains unclear. In this study, using both in vivo and in vitro models, we report that the level of β-catenin is positively correlated to the degree of renal interstitial fibrosis (RIF). Protein phosphatase 2A (PP2A) binds to β-catenin and suppresses its phosphorylation, thereby increasing the total β-catenin expression. Additionally, NCTD dramatically elevates the level of phosphorylated β-catenin and decreases total β-catenin expression in a dose-dependent manner, consequently leading to the reduction of RIF. Mechanistically, PP2A-mediated suppression of β-catenin phosphorylation is an essential target for the anti-renal interstitial fibrotic effect of NCTD. Therefore, we report a potential theoretical basis for clinical application of NCTD in treating RIF.






Similar content being viewed by others
Abbreviations
- NCTD:
-
Norcantharidin
- RIF:
-
Renal interstitial fibrosis
- PP2A:
-
Protein phosphatase 2A
- UUO:
-
Unilateral ureter obstruction
- HK-2 cells:
-
Human proximal tubular cells
- Col-I:
-
Collagen type I
- CKD:
-
Chronic kidney disease
- TGF-β1:
-
Transforming growth factor-beta1
- OA:
-
Okadaic acid
- ECM:
-
Extracellular matrix
References
Liu, Z.H. 2013. Nephrology in China. Nature Reviews. Nephrology 9: 523–528.
Webster, A.C., E.V. Nagler, R.L. Morton, and P. Masson. 2017. Chronic kidney disease. Lancet 389: 1238–1252.
Genovese, F., A.A. Manresa, D.J. Leeming, M.A. Karsdal, and P. Boor. 2014. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis & Tissue Repair 7: 4.
Liu, Y. 2011. Cellular and molecular mechanisms of renal fibrosis. Nature Reviews. Nephrology 7: 684–696.
Dkhil, M.A., R.B. Kassab, S. Al-Quraishy, M.M. Abdel-Daim, R. Zrieq, and A.E. Abdel Moneim. 2018. Ziziphus spina-christi (L.) leaf extract alleviates myocardial and renal dysfunction associated with sepsis in mice. Biomedicine & Pharmacotherapy 102: 64–75.
Abdel-Daim, M.M., Y.S. El-Sayed, M.A. Eldaim, and A. Ibrahim. 2017. Nephroprotective efficacy of ceftriaxone against cisplatin-induced subchronic renal fibrosis in rats. Naunyn-Schmiedeberg's Archives of Pharmacology 390: 301–309.
Ibrahim, A., M.A. Eldaim, and M.M. Abdel-Daim. 2016. Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology 68: 1039–1048.
Abdel-Daim, M.M., and A. El-Ghoneimy. 2015. Synergistic protective effects of ceftriaxone and ascorbic acid against subacute deltamethrin-induced nephrotoxicity in rats. Renal Failure 37: 297–304.
Abdel-Daim, M., B.E. El-Bialy, H.G. Rahman, A.M. Radi, H.A. Hefny, and A.M. Hassan. 2016. Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: Biochemical and histopathological studies. Biomedicine & Pharmacotherapy 77: 79–85.
Zeisberg, M., and E.G. Neilson. 2010. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834.
Kadioglu, O., N.S. Kermani, G. Kelter, U. Schumacher, H.H. Fiebig, H.J. Greten, and T. Efferth. 2014. Pharmacogenomics of cantharidin in tumor cells. Biochemical Pharmacology 87: 399–409.
Zhang, L., et al. 2013. Norcantharidin inhibits tumor angiogenesis via blocking VEGFR2/MEK/ERK signaling pathways. Cancer Science 104: 604–610.
Li, Y., et al. 2011. Norcantharidin attenuates tubulointerstitial fibrosis in rat models with diabetic nephropathy. Renal Failure 33: 233–241.
Liu, F.Y., et al. 2008. Norcantharidin ameliorates proteinuria, associated tubulointerstitial inflammation and fibrosis in protein overload nephropathy. American Journal of Nephrology 28: 465–477.
Li, Y., et al. 2013. Norcantharidin inhibits renal interstitial fibrosis by blocking the tubular epithelial-mesenchymal transition. PLoS One 8: e66356.
Liu, X.H., et al. 1995. Effects of norcantharidin, a protein phosphatase type-2A inhibitor, on the growth of normal and malignant haemopoietic cells. European Journal of Cancer 31a: 953–963.
O'Connor, C.M., A. Perl, D. Leonard, J. Sangodkar, and G. Narla. 2018. Therapeutic targeting of PP2A. The International Journal of Biochemistry & Cell Biology 96: 182–193.
Hou, T., et al. 2015. Norcantharidin inhibits renal interstitial fibrosis by downregulating PP2Ac expression. American Journal of Translational Research 7: 2199–2211.
Wang, Y., C.J. Zhou, and Y. Liu. 2018. Wnt signaling in kidney development and disease. Progress in Molecular Biology and Translational Science 153: 181–207.
Sagredo, A.I., et al. 2018. TRPM4 regulates Akt/GSK3-beta activity and enhances beta-catenin signaling and cell proliferation in prostate cancer cells. Molecular Oncology 12: 151–165.
Cai, T., D. Sun, Y. Duan, Y. Qiu, C. Dai, J. Yang, and W. He. 2018. FHL2 promotes tubular epithelial-to-mesenchymal transition through modulating beta-catenin signalling. Journal of Cellular and Molecular Medicine 22: 1684–1695.
Hao, S., et al. 2011. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 22: 1642–1653.
Li, M., H. Zhou, J. Di, M. Yang, and F. Jia. 2019. ILK participates in renal interstitial fibrosis by altering the phenotype of renal tubular epithelial cells via TGF-beta1/smad pathway. European Review for Medical and Pharmacological Sciences 23: 289–296.
Wang, M., et al. 2018. Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/beta-catenin pathway against renal fibrosis. British Journal of Pharmacology 175: 2689–2708.
Yao, L., et al. 2015. High phosphorus level leads to aortic calcification via beta-catenin in chronic kidney disease. American Journal of Nephrology 41: 28–36.
Yu, N., et al. 2015. HSP105 recruits protein phosphatase 2A to dephosphorylate beta-catenin. Molecular and Cellular Biology 35: 1390–1400.
Maheshwari, S., et al. 2017. Discovery of a novel small-molecule inhibitor that targets PP2A-beta-catenin signaling and restricts tumor growth and metastasis. Molecular Cancer Therapeutics 16: 1791–1805.
Lavoz, C., Y.S. Matus, M. Orejudo, J.D. Carpio, A. Droguett, J. Egido, S. Mezzano, and M. Ruiz-Ortega. 2019. Interleukin-17A blockade reduces albuminuria and kidney injury in an accelerated model of diabetic nephropathy. Kidney International 95: 1418–1432.
Garcia-Sanchez, O., F.J. Lopez-Hernandez, and J.M. Lopez-Novoa. 2010. An integrative view on the role of TGF-beta in the progressive tubular deletion associated with chronic kidney disease. Kidney International 77: 950–955.
Li, Y., et al. 2011. Norcantharidin inhibits the expression of extracellular matrix and TGF-beta1 in HK-2 cells induced by high glucose independent of calcineurin signal pathway. Laboratory Investigation 91: 1706–1716.
Tan, R.J., D. Zhou, L. Zhou, and Y. Liu. 2014. Wnt/beta-catenin signaling and kidney fibrosis. Kidney International. Supplement (2011 4: 84–90.
Matsuyama, M., A. Nomori, K. Nakakuni, A. Shimono, and M. Fukushima. 2014. Secreted frizzled-related protein 1 (Sfrp1) regulates the progression of renal fibrosis in a mouse model of obstructive nephropathy. The Journal of Biological Chemistry 289: 31526–31533.
Conduit, S.E., S. Hakim, S.J. Feeney, L.M. Ooms, J.M. Dyson, H.E. Abud, and C.A. Mitchell. 2019. Beta-catenin ablation exacerbates polycystic kidney disease progression. Human Molecular Genetics 28: 230–244.
Wang, X.D., X.F. Huang, Q.R. Yan, and C.D. Bao. 2014. Aberrant activation of the WNT/beta-catenin signaling pathway in lupus nephritis. PLoS One 9: e84852.
Huang, X., H. Xue, J. Ma, Y. Zhang, J. Zhang, Y. Liu, X. Qin, and C. Sun. 2019. Salidroside ameliorates Adriamycin nephropathy in mice by inhibiting beta-catenin activity. Journal of Cellular and Molecular Medicine 23: 4443–4453.
Duan, S., Y. Wu, C. Zhao, M. Chen, Y. Yuan, C. Xing, and B. Zhang. 2016. The wnt/beta-catenin signaling pathway participates in rhein ameliorating kidney injury in DN mice. Molecular and Cellular Biochemistry 411: 73–82.
White, K.A., B.K. Grillo-Hill, M. Esquivel, J. Peralta, V.N. Bui, I. Chire, and D.L. Barber. 2018. Beta-catenin is a pH sensor with decreased stability at higher intracellular pH. The Journal of Cell Biology 217: 3965–3976.
Thompson, J.J., and C.S. Williams. 2018. Protein phosphatase 2A in the regulation of Wnt signaling, stem cells, and cancer. Genes (Basel) 9.
Mitra, A., M.E. Menezes, L.K. Pannell, M.S. Mulekar, R.E. Honkanen, L.A. Shevde, and R.S. Samant. 2012. DNAJB6 chaperones PP2A mediated dephosphorylation of GSK3beta to downregulate beta-catenin transcription target, osteopontin. Oncogene 31: 4472–4483.
Carmen Figueroa-Aldariz, M., M.C. Castaneda-Patlan, P. Santoyo-Ramos, A. Zentella, and M. Robles-Flores. 2015. Protein phosphatase 2A is essential to maintain active Wnt signaling and its Abeta tumor suppressor subunit is not expressed in colon cancer cells. Molecular Carcinogenesis 54: 1430–1441.
Deng, L., J. Dong, and W. Wang. 2013. Exploiting protein phosphatase inhibitors based on cantharidin analogues for cancer drug discovery. Mini Reviews in Medicinal Chemistry 13: 1166–1176.
Deng, L., S. Tang, and Q. Qian. 2012. A review for mini-review in medicinal chemistry. Mini Reviews in Medicinal Chemistry.
Xie, D., J. Xie, Y. Wan, L. Ma, X. Qi, K. Wang, and S. Yang. 2016. Norcantharidin blocks Wnt/beta-catenin signaling via promoter demethylation of WIF-1 in glioma. Oncology Reports 35: 2191–2197.
Lu, S., Y. Gao, X. Huang, and X. Wang. 2014. Cantharidin exerts anti-hepatocellular carcinoma by miR-214 modulating macrophage polarization. International Journal of Biological Sciences 10: 415–425.
Xie, J., Y. Zhang, X. Hu, R. Lv, D. Xiao, L. Jiang, and Q. Bao. 2015. Norcantharidin inhibits Wnt signal pathway via promoter demethylation of WIF-1 in human non-small cell lung cancer. Medical Oncology 32: 145.
Acknowledgments
This study was supported by the Natural Science Foundation of China (Grant No. 81100486 and 81370792), the Hunan Provincial Science and Technology Program of China (Grant No. 2017SK2072), and the Changsha Science and Technology Program of China (Grant No. kq1901122).
Author information
Authors and Affiliations
Contributions
Zheng Xiao, Lu Wen, and Ying Li designed the experiments. Zheng Xiao wrote the manuscript. Ying Li provided the experimental materials. Zheng Xiao and Dong Zeng performed the experiments. Zheng Xiao, Lu Wen, and Dandan Yin performed the data analysis. Xun Zhou and Chengyuan Tang revised and put valuable inputs in the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiao, Z., Wen, L., Zeng, D. et al. Protein Phosphatase 2A Inhibiting β-Catenin Phosphorylation Contributes Critically to the Anti-renal Interstitial Fibrotic Effect of Norcantharidin. Inflammation 43, 878–891 (2020). https://doi.org/10.1007/s10753-019-01173-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01173-0