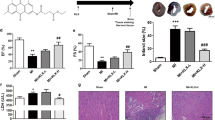
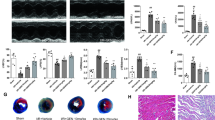
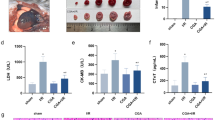
Abstract
Isofraxidin is a well-known coumarin compound refined from traditional Chinese medicines. It has been previously demonstrated to play an anti-inflammatory role in various inflammatory conditions. However, the effect of isofraxidin on myocardial infarction (MI) remains uncovered. In this study, we aimed to investigate the effect of isofraxidin on MI. MI mice was created and triphenyltetrazolium chloride (TTC) staining as well as echocardiographic evaluation were conducted to analyze the severity of MI. Oxygen-glucose deprivation (OGD) was used for the mimics of ischemic stress in murine cardiomyocytes, and Cell Counting Kit-8 (CCK-8), Annexin V, and lactate dehydrogenase (LDH) release assays were conducted for cell viability. Western blot was used for the detection of NOD-like receptor family, pyrin domain containing 3 (NLRP3), and adapter protein apoptosis-associated speck-like protein (ASC) in heart tissues and cardiomyocytes. Real-time polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA) were applied for the detection of proinflammatory cytokines. We found that isofraxidin alleviated the severity of MI and produced a cardio-protective effect against OGD damage. Isofraxidin also decreased the overall and local inflammatory reaction in MI. Those effects were through the inhibition of the NLRP3 inflammasome. Taken together, we initially reported the cardio-protective and alleviative effect of isofraxidin on MI and uncovered its underlying mechanism related to the NLRP3 inflammasome inhibition.






Similar content being viewed by others
References
Raber, I., C.P. McCarthy, M. Vaduganathan, D.L. Bhatt, D.A. Wood, J.G.F. Cleland, R.S. Blumenthal, and J.W. McEvoy. 2019. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet 393 (10186): 2155–2167. https://doi.org/10.1016/S0140-6736(19)30541-0.
Oder, D., M.S. Topp, and P. Nordbeck. 2019. Coronary B-cell lymphoma infiltration causing myocardial infarction. European Heart Journal. https://doi.org/10.1093/eurheartj/ehz538.
Koechlin, L., J. Boeddinghaus, T. Nestelberger, D. Wussler, J. Walter, F.J. Martin-Sanchez, N. Geigy, et al. 2019. Early diagnosis of myocardial infarction in patients with a history of coronary artery bypass grafting. Journal of the American College of Cardiology 74 (4): 587–589. https://doi.org/10.1016/j.jacc.2019.05.044.
Lu, L., M. Liu, R. Sun, Y. Zheng, and P. Zhang. 2015. Myocardial infarction: Symptoms and treatments. Cell Biochemistry and Biophysics 72 (3): 865–867. https://doi.org/10.1007/s12013-015-0553-4.
Kim, C.Y., J.H. Lee, S.Y. Jang, M.H. Bae, D.H. Yang, H.S. Park, Y. Cho, et al. 2019. Usefulness of calculation of cardiovascular risk factors to predict outcomes in patients with acute myocardial infarction. The American Journal of Cardiology. https://doi.org/10.1016/j.amjcard.2019.06.010.
Siri, S.R.A., B.M. Eliassen, B.K. Jacobsen, M. Melhus, A.R. Broderstad, V.L. Michalsen, and T. Braaten. 2019. Changes in conventional cardiovascular risk factors and the estimated 10-year risk of acute myocardial infarction or cerebral stroke in Sami and non-Sami populations in two population-based cross-sectional surveys: The SAMINOR study. BMJ Open 9 (7): e028939. https://doi.org/10.1136/bmjopen-2019-028939.
Sigirci, S., S.S. Yildiz, K. Keskin, G. Cetinkal, G. Aksan, A. Gurdal, S. Cetin, H. Kilci, and K.O. Kilickesmez. 2019. The predictive value of stress hyperglycemia on thrombus burden in nondiabetic patients with ST-segment elevation myocardial infarction. Blood Coagulation & Fibrinolysis. https://doi.org/10.1097/MBC.0000000000000832.
Rauf, A., M. Shah, D.M. Yellon, and S.M. Davidson. 2019. The role of caspase 1 in ischemia/reperfusion injury of the myocardium. Journal of Cardiovascular Pharmacology. https://doi.org/10.1097/FJC.0000000000000694.
Ibanez, B., A.H. Aletras, A.E. Arai, H. Arheden, J. Bax, C. Berry, C. Bucciarelli-Ducci, et al. 2019. Cardiac MRI endpoints in myocardial infarction experimental and clinical trials: JACC scientific expert panel. Journal of the American College of Cardiology 74 (2): 238–256. https://doi.org/10.1016/j.jacc.2019.05.024.
Hu, J., C.X. Huang, P.P. Rao, G.Q. Cao, Y. Zhang, J.P. Zhou, L.Y. Zhu, M.X. Liu, and G.G. Zhang. 2019. MicroRNA-155 inhibition attenuates endoplasmic reticulum stress-induced cardiomyocyte apoptosis following myocardial infarction via reducing macrophage inflammation. European Journal of Pharmacology 857: 172449. https://doi.org/10.1016/j.ejphar.2019.172449.
Felger, J.C. 2018. Imaging the role of inflammation in mood and anxiety-related disorders. Current Neuropharmacology 16 (5): 533–558. https://doi.org/10.2174/1570159X15666171123201142.
Ablasser, A., and Z.J. Chen. 2019. cGAS in action: Expanding roles in immunity and inflammation. Science 363 (6431). https://doi.org/10.1126/science.aat8657.
Gong, T., W. Jiang, and R. Zhou. 2018. Control of inflammasome activation by phosphorylation. Trends in Biochemical Sciences 43 (9): 685–699. https://doi.org/10.1016/j.tibs.2018.06.008.
Shin, J.I., K.H. Lee, Y.H. Joo, J.M. Lee, J. Jeon, H.J. Jung, M. Shin, et al. 2019. Inflammasomes and autoimmune and rheumatic diseases: A comprehensive review. J Autoimmun: 102299. https://doi.org/10.1016/j.jaut.2019.06.010.
Winsor, N., C. Krustev, J. Bruce, D.J. Philpott, and S.E. Girardin. 2019. Canonical and noncanonical inflammasomes in intestinal epithelial cells. Cell Microbiol: e13079. https://doi.org/10.1111/cmi.13079.
Henderson, J., S. Bhattacharyya, J. Varga, and S. O’Reilly. 2018. Targeting TLRs and the inflammasome in systemic sclerosis. Pharmacology & Therapeutics 192: 163–169. https://doi.org/10.1016/j.pharmthera.2018.08.003.
An, N., Y. Gao, Z. Si, H. Zhang, L. Wang, C. Tian, M. Yuan, et al. 2019. Regulatory mechanisms of the NLRP3 inflammasome, a novel immune-inflammatory marker in cardiovascular diseases. Frontiers in Immunology 10: 1592. https://doi.org/10.3389/fimmu.2019.01592.
Kelley, N., D. Jeltema, Y. Duan, and Y. He. 2019. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. International Journal of Molecular Sciences 20 (13). https://doi.org/10.3390/ijms20133328.
Shao, B.Z., Z.Q. Xu, B.Z. Han, D.F. Su, and C. Liu. 2015. NLRP3 inflammasome and its inhibitors: A review. Frontiers in Pharmacology 6: 262. https://doi.org/10.3389/fphar.2015.00262.
Toldo, S., and A. Abbate. 2018. The NLRP3 inflammasome in acute myocardial infarction. Nature Reviews. Cardiology 15 (4): 203–214. https://doi.org/10.1038/nrcardio.2017.161.
Gao, R., H. Shi, S. Chang, Y. Gao, X. Li, C. Lv, H. Yang, H. Xiang, J. Yang, L. Xu, and Y. Tang. 2019. The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. International Immunopharmacology 74: 105575. https://doi.org/10.1016/j.intimp.2019.04.022.
Bian, Y., X. Li, P. Pang, X.L. Hu, S.T. Yu, Y.N. Liu, X. Li, N. Wang, J.H. Wang, W. Xiao, W.J. du, and B.F. Yang. 2019. Kanglexin, a novel anthraquinone compound, protects against myocardial ischemic injury in mice by suppressing NLRP3 and pyroptosis. Acta Pharmacologica Sinica: 1–8. https://doi.org/10.1038/s41401-019-0307-8.
Yamazaki, T., and T. Tokiwa. 2010. Isofraxidin, a coumarin component from Acanthopanax senticosus, inhibits matrix metalloproteinase-7 expression and cell invasion of human hepatoma cells. Biological & Pharmaceutical Bulletin 33 (10): 1716–1722. https://doi.org/10.1248/bpb.33.1716.
Su, X., B. Liu, F. Gong, J. Yin, Q. Sun, Y. Gao, Z. Lv, and X. Wang. 2019. Isofraxidin attenuates IL-1beta-induced inflammatory response in human nucleus pulposus cells. Journal of Cellular Biochemistry 120 (8): 13302–13309. https://doi.org/10.1002/jcb.28604.
Li, J., X. Li, Z. Li, L. Zhang, Y. Liu, H. Ding, and S. Yin. 2017. Isofraxidin, a coumarin component improves high-fat diet induced hepatic lipid homeostasis disorder and macrophage inflammation in mice. Food & Function 8 (8): 2886–2896. https://doi.org/10.1039/c7fo00290d.
Lin, J., X. Li, W. Qi, Y. Yan, K. Chen, X. Xue, X. Xu, Z. Feng, and X. Pan. 2018. Isofraxidin inhibits interleukin-1beta induced inflammatory response in human osteoarthritis chondrocytes. International Immunopharmacology 64: 238–245. https://doi.org/10.1016/j.intimp.2018.09.003.
Liu, L., Q. Mu, W. Li, W. Xing, H. Zhang, T. Fan, H. Yao, and L. He. 2015. Isofraxidin protects mice from LPS challenge by inhibiting pro-inflammatory cytokines and alleviating histopathological changes. Immunobiology 220 (3): 406–413. https://doi.org/10.1016/j.imbio.2014.10.007.
Liao, Q., S. Qu, L.X. Tang, L.P. Li, D.F. He, C.Y. Zeng, and W.E. Wang. 2019. Irisin exerts a therapeutic effect against myocardial infarction via promoting angiogenesis. Acta Pharmacologica Sinica 40: 1314–1321. https://doi.org/10.1038/s41401-019-0230-z.
Yu, W., G. Jin, J. Zhang, and W. Wei. 2019. Selective activation of cannabinoid receptor 2 attenuates myocardial infarction via suppressing NLRP3 inflammasome. Inflammation 42 (3): 904–914. https://doi.org/10.1007/s10753-018-0945-x.
Niu, X., W. Xing, W. Li, T. Fan, H. Hu, and Y. Li. 2012. Isofraxidin exhibited anti-inflammatory effects in vivo and inhibited TNF-alpha production in LPS-induced mouse peritoneal macrophages in vitro via the MAPK pathway. International Immunopharmacology 14 (2): 164–171. https://doi.org/10.1016/j.intimp.2012.06.022.
Niu, X., Y. Wang, W. Li, Q. Mu, H. Li, H. Yao, and H. Zhang. 2015. Protective effects of isofraxidin against lipopolysaccharide-induced acute lung injury in mice. International Immunopharmacology 24 (2): 432–439. https://doi.org/10.1016/j.intimp.2014.12.041.
Takahashi, M. 2019. Role of NLRP3 inflammasome in cardiac inflammation and remodeling after myocardial infarction. Biological & Pharmaceutical Bulletin 42 (4): 518–523. https://doi.org/10.1248/bpb.b18-00369.
Funding
This work was supported by a Zhejiang Provincial Medicine Health Science and Technology Program (No. 2018ZB057).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Fig. 1
. Isofraxidin increased the levels of proinflammatory cytokines in MI mice models and cardiomyocytes under the challenge of OGD. (a,b) The left anterior descending coronary artery was occluded for the creation of MI mice models. Mice with mere thoracotomy were treated as the sham group. Isofraxidin at the dose of 20 mg/kg body weight was intraperitoneally given to mice at 5 min before occlusion. Saline in the equal volume was intraperitoneally injected as vehicle. Quantitative analysis of IL-4 and IL-10 in serum at 6 h after occlusion (n=6 per group). **P<0.01 vs. sham group; ##P<0.01 vs. MI+Vehicle group; data are presented as mean±SEM. (c,d) Primary cardiomyocytes were harvested from mice and challenged with OGD for 4 h. Isofraxidin at the dose of 100 μM was treated at 10 min before suffering OGD. PBS in the equal volume was given as vehicle. Quantitative analysis of supernatant IL-4 and IL-10 (n=6 per group). **P<0.01 vs. normal group; ##P<0.01 vs. OGD+Vehicle group; data are presented as mean±SEM. (PNG 247 kb)
Rights and permissions
About this article
Cite this article
Chen, G., Song, X., Lin, D. et al. Isofraxidin Alleviates Myocardial Infarction Through NLRP3 Inflammasome Inhibition. Inflammation 43, 712–721 (2020). https://doi.org/10.1007/s10753-019-01158-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01158-z