Abstract
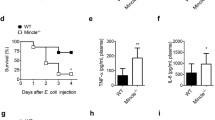
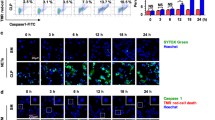
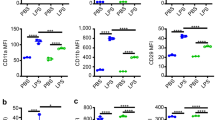
As a novel cytokine, cytokine-like 1 (CYTL1) is a classical secretory protein, and its potential biological function remains to be determined. In this study, we found that expression of CYTL1 was upregulated in neutrophils upon inflammatory stimuli. We demonstrated that CYTL1 enhanced phagocytosis of Escherichia coli by activated neutrophils both in vivo and in vitro through phosphorylation of protein kinase B (Akt). CYTL1-induced chemotactic activity in lipopolysaccharide (LPS) stimulated neutrophils, and the mechanism may be related to CC chemokine receptor 2 (CCR2) mediated action. CYTL1 also increased the release of reactive oxygen species (ROS) in LPS-stimulated neutrophils. These data indicate that upon inflammatory stimulation, neutrophil-derived CYTL1 may play a crucial role in the activation of neutrophils during pathogenic infections.






Similar content being viewed by others
References
Singer, M., C.S. Deutschman, C.W. Seymour, M. Shankar-Hari, D. Annane, M. Bauer, R. Bellomo, G.R. Bernard, J.D. Chiche, C.M. Coopersmith, R.S. Hotchkiss, M.M. Levy, J.C. Marshall, G.S. Martin, S.M. Opal, G.D. Rubenfeld, T. van der Poll, J.L. Vincent, and D.C. Angus. 2016. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801–810. https://doi.org/10.1001/jama.2016.0287.
Alcamo, A.M., D. Pang, D.A. Bashir, J.A. Carcillo, T.C. Nguyen, and R.K. Aneja. 2019. Role of damage-associated molecular patterns and uncontrolled inflammation in pediatric sepsis-induced multiple organ dysfunction syndrome. J Pediatr Intensive Care 8 (1): 25–31. https://doi.org/10.1055/s-0038-1675639.
Cawcutt, K.A., and S.G. Peters. 2014. Severe sepsis and septic shock: clinical overview and update on management. Mayo Clinic Proceedings 89: 1572–1578. https://doi.org/10.1016/j.mayocp.2014.07.009.
De La Rica, Suarez, A.F. Gilsanz, and E. Maseda. 2016. Epidemiologic trends of sepsis in western countries. Ann Transl Med 4: 325. https://doi.org/10.21037/atm.2016.08.59.
Minasyan, H. 2019. Sepsis: mechanisms of bacterial injury to the patient. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 27: 19. https://doi.org/10.1186/s13049-019-0596-4.
Shen, X.F., K. Cao, J.P. Jiang, W.X. Guan, and J.F. Du. 2017. Neutrophil dysregulation during sepsis: an overview and update. Journal of Cellular and Molecular Medicine 21: 1687–1697. https://doi.org/10.1111/jcmm.13112.
Teng, T.S., A.L. Ji, X.Y. Ji, and Y.Z. Li. 2017. Neutrophils and immunity: from bactericidal action to being conquered. Journal of Immunology Research 2017: 9671604. https://doi.org/10.1155/2017/9671604.
Cavaillon, J.M. 2018. Exotoxins and endotoxins: inducers of inflammatory cytokines. Toxicon 149: 45–53. https://doi.org/10.1016/j.toxicon.2017.10.016.
Sonego, F., F.V. Castanheira, R.G. Ferreira, A. Kanashiro, C.A. Leite, D.C. Nascimento, D.F. Colon, F. Borges Vde, J.C. Alves-Filho, and F.Q. Cunha. 2016. Paradoxical roles of the neutrophil in sepsis: protective and deleterious. Frontiers in Immunology 7: 155. https://doi.org/10.3389/fimmu.2016.00155.
Lerman, Y.V., and M. Kim. 2015. Neutrophil migration under normal and sepsis conditions. Cardiovascular & Hematological Disorders Drug Targets 15: 19–28. https://doi.org/10.2174/1871529X15666150108113236.
Kuzmich, N.N., K.V. Sivak, V.N. Chubarev, Y.B. Porozov, T.N. Savateeva-Lyubimova, and F. Peri. 2017. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines (Basel) 5: 34. https://doi.org/10.3390/vaccines5040034.
Tomczak, A., K. Singh, A.G. Gittis, J. Lee, D.N. Garboczi, and P.M. Murphy. 2017. Biochemical and biophysical characterization of cytokine-like protein 1 (CYTL1). Cytokine 96: 238–246. https://doi.org/10.1016/j.cyto.2017.04.023.
Zhu, S., V. Kuek, S. Bennett, H. Xu, V. Rosen, and J. Xu. 2019. Protein Cytl1: its role in chondrogenesis, cartilage homeostasis, and disease. Cellular and Molecular Life Sciences 76: 3515–3523. https://doi.org/10.1007/s00018-019-03137-x.
Jeon, J., H. Oh, G. Lee, J.H. Ryu, J. Rhee, J.H. Kim, K.H. Chung, W.K. Song, C.H. Chun, and J.S. Chun. 2011. Cytokine-like 1 knock-out mice (Cytl1(−/−)) show normal cartilage and bone development but exhibit augmented osteoarthritic cartilage destruction. Journal of Biological Chemistry 286: 27206–27213. https://doi.org/10.1074/jbc.M111.218065.
Chao, C., B. Joyce-Shaikh, J. Grein, M. Moshrefi, F. Raoufi, D.M. Laface, T.K. McClanahan, et al. 2011. C17 prevents inflammatory arthritis and associated joint destruction in mice. PLoS One 6: e22256. https://doi.org/10.1371/journal.pone.0022256.
Wang, X., T. Li, W. Wang, W. Yuan, H. Liu, Y. Cheng, P. Wang, Y. Zhang, and W. Han. 2016. Cytokine-like 1 chemoattracts monocytes/macrophages via CCR2. Journal of Immunology 196: 4090–4099. https://doi.org/10.4049/jimmunol.1501908.
Kim, J., J. Kim, S.H. Lee, S.V. Kepreotis, J. Yoo, J.S. Chun, R.J. Hajjar, D. Jeong, and W.J. Park. 2016. Cytokine-like 1 regulates cardiac fibrosis via modulation of TGF-beta signaling. PLoS One 11: e0166480. https://doi.org/10.1371/journal.pone.0166480.
Begley, L.A., S. Kasina, J. MacDonald, and J.A. Macoska. 2008. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine 43: 194–199. https://doi.org/10.1016/j.cyto.2008.05.012.
Wen, M.J., H.M. Wang, X.L. Zhang, J. Long, Z. Lv, Q.L. Kong, and Y.Q. An. 2012. Cytokine-like 1 is involved in the growth and metastasis of neuroblastoma cells. International Journal of Oncology 41: 1419–1424. https://doi.org/10.3892/ijo.2012.1552.
Kwon, Y.J., S.J. Lee, J.S. Koh, S.H. Kim, H.W. Lee, M.C. Kang, J.B. Bae, Y.J. Kim, and J.H. Park. 2012. Genome-wide analysis of DNA methylation and the gene expression change in lung cancer. Journal of Thoracic Oncology 7: 20–33. https://doi.org/10.1097/JTO.0b013e3182307f62.
Tanskanen, T., A.E. Gylfe, R. Katainen, M. Taipale, L. Renkonen-Sinisalo, H. Jarvinen, J.P. Mecklin, et al. 2015. Systematic search for rare variants in Finnish early-onset colorectal cancer patients. Cancer Genetics 208: 35–40. https://doi.org/10.1016/j.cancergen.2014.12.004.
Wichterman, Keith A., Arthur E. Baue, and Irshad H. Chaudry. 1980. Sepsis and septic shock—a review of laboratory models and a proposal. Journal of Surgical Research 29: 189–201. https://doi.org/10.1016/0022-4804(80)90037-2.
Souto, F.O., J.C. Alves-Filho, W.M. Turato, M. Auxiliadora-Martins, A. Basile-Filho, and F.Q. Cunha. 2011. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. American Journal of Respiratory and Critical Care Medicine 183: 234–242. https://doi.org/10.1164/rccm.201003-0416OC.
Wang, W.Y., T. Li, X.L. Wang, W.X. Yuan, Y.Y. Cheng, H.Y. Zhang, E.Q. Xu, et al. 2015. FAM19A4 is a novel cytokine ligand of formyl peptide receptor 1 (FPR1) and is able to promote the migration and phagocytosis of macrophages. Cellular & Molecular Immunology 12: 615–624. https://doi.org/10.1038/cmi.2014.61.
Chousterman, B.G., F.K. Swirski, and G.F. Weber. 2017. Cytokine storm and sepsis disease pathogenesis. Seminars in Immunopathology 39: 517–528. https://doi.org/10.1007/s00281-017-0639-8.
Petri, B., and M.J. Sanz. 2018. Neutrophil chemotaxis. Cell and Tissue Research 371: 425–436. https://doi.org/10.1007/s00441-017-2776-8.
Degryse, B., and M. de Virgilio. 2003. The nuclear protein HMGB1, a new kind of chemokine? FEBS Letters 553: 11–17. https://doi.org/10.1016/s0014-5793(03)01027-5.
van der Vorst, E.P., Y. Doring, and C. Weber. 2015. MIF and CXCL12 in cardiovascular diseases: functional differences and similarities. Frontiers in Immunology 6: 373. https://doi.org/10.3389/fimmu.2015.00373.
Bose, S., and J. Cho. 2013. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Archives of Pharmacal Research 36: 1039–1050. https://doi.org/10.1007/s12272-013-0161-z.
Raghu, H., C.M. Lepus, Q. Wang, H.H. Wong, N. Lingampalli, F. Oliviero, L. Punzi, N.J. Giori, S.B. Goodman, C.R. Chu, J.B. Sokolove, and W.H. Robinson. 2017. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Annals of the Rheumatic Diseases 76: 914–922. https://doi.org/10.1136/annrheumdis-2016-210426.
Winter, C., C. Silvestre-Roig, A. Ortega-Gomez, P. Lemnitzer, H. Poelman, A. Schumski, J. Winter, et al. 2018. Chrono-pharmacological targeting of the CCL2-CCR2 axis ameliorates atherosclerosis. Cell Metabolism 28 (175–182): e175. https://doi.org/10.1016/j.cmet.2018.05.002.
Tomczak, A., and M.T. Pisabarro. 2011. Identification of CCR2-binding features in Cytl1 by a CCL2-like chemokine model. Proteins 79: 1277–1292. https://doi.org/10.1002/prot.22963.
Kapoor A Fau - Thiemermann, Christoph, and C. Thiemermann. 2011. Targeting CCR2: a novel therapeutic strategy for septic shock? American Journal of Respiratory and Critical Care Medicine 183: 150–151. doi: https://doi.org/10.1164/rccm.201009-1403ED.
Fang, H., W. Jiang, J. Cheng, Y. Lu, A. Liu, L. Kan, and U. Dahmen. 2015. Balancing innate immunity and inflammatory state via modulation of neutrophil function: a novel strategy to fight sepsis. Journal of Immunology Research 2015: 187048. https://doi.org/10.1155/2015/187048.
Venet, F., and G. Monneret. 2018. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nature Reviews. Nephrology 14: 121–137. https://doi.org/10.1038/nrneph.2017.165.
Moussion, C., Jean-Philippe Ortega N Fau - Girard, and J. P. Girard. 2008. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One 3: e3331. doi: https://doi.org/10.1371/journal.pone.0003331.
Cohen, I., P. Rider, Y. Carmi, A. Braiman, S. Dotan, M.R. White, E. Voronov, M.U. Martin, C.A. Dinarello, and R.N. Apte. 2010. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proceedings of the National Academy of Sciences of the United States of America 107: 2574–2579. https://doi.org/10.1073/pnas.0915018107.
Zaki, O.S., M.M. Safar, A.A. Ain-Shoka, and L.A. Rashed. 2018. Bone marrow mesenchymal stem cells combat lipopolysaccharide-induced sepsis in rats via amendment of P38-MAPK signaling cascade. Inflammation 41: 541–554. https://doi.org/10.1007/s10753-017-0710-6.
Wrann, C.D., S.W. Winter, T. Barkhausen, F. Hildebrand, C. Krettek, and N.C. Riedemann. 2007. Distinct involvement of p38-, ERK1/2 and PKC signaling pathways in C5a-mediated priming of oxidative burst in phagocytic cells. Cellular Immunology 245: 63–69. https://doi.org/10.1016/j.cellimm.2007.04.001.
Oliveira, J.S.S., G.D.S. Santos, J.A. Moraes, A.M. Saliba, T.C. Barja-Fidalgo, A.L. Mattos-Guaraldi, and P.E. Nagao. 2018. Reactive oxygen species generation mediated by NADPH oxidase and PI3K/Akt pathways contribute to invasion of Streptococcus agalactiae in human endothelial cells. Memórias do Instituto Oswaldo Cruz 113: e140421. https://doi.org/10.1590/0074-02760170421.
Schneller, D., R. Hofer-Warbinek, C. Sturtzel, K. Lipnik, B. Gencelli, M. Seltenhammer, M.J. Wen, et al. 2019. Cytokine-like 1 is a novel proangiogenic factor secreted by and mediating functions of endothelial progenitor cells. Circulation Research 124: 243–255. https://doi.org/10.1161/Circresaha.118.313645.
Shin, Y., Y. Won, J.I. Yang, and J.S. Chun. 2019. CYTL1 regulates bone homeostasis in mice by modulating osteogenesis of mesenchymal stem cells and osteoclastogenesis of bone marrow-derived macrophages. Cell Death & Disease 10: 47. https://doi.org/10.1038/s41419-018-1284-4.
Acknowledgments
We thank Professor Han Wenling from the Department of Immunology, Peking University Health Science Center for providing the CYTL1 protein.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal experimental procedures were approved by the Ethics Committee of the Peking University People’s Hospital.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, H., Li, S., Zhao, X. et al. CYTL1 Promotes the Activation of Neutrophils in a Sepsis Model. Inflammation 43, 274–285 (2020). https://doi.org/10.1007/s10753-019-01116-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01116-9