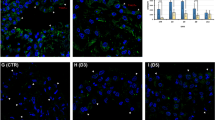
Abstract
In our previous work, we showed that during inflammation-induced epithelial-to-mesenchymal transition (EMT), mesenteric mesothelial cells express ED1 (pan-macrophage marker), indicating that they are transformed into macrophage-like cells. In this paper, we provide additional evidences about this transition by following the phagocytic activity and the TNFα production of mesenteric mesothelial cells during inflammation. Upon injection of India ink particles or fluorescent-labeled bioparticles (pHrodo) into the peritoneal cavity of rats pretreated with Freund‘s adjuvant, we found that mesothelial cells efficiently engulfed these particles. A similar increase of internalization could be observed by mesothelial cells in GM-CSF pretreated primary mesenteric culture. Since macrophages are the major producers of tumor necrosis factor, TNFα, we investigated expression level of TNFα during inflammation-induced EMT and found that TNFα was indeed expressed in these cells, reaching the highest level at the 5th day of inflammation. Since TNFα is one of the target genes of early growth response (EGR1) transcription factor, playing important role in monocyte-macrophage differentiation, expression of EGR1 in mesothelial cells was also investigated by Western blot and immunocytochemistry. While mesothelial cells did not express EGR1, a marked increase was observed in mesothelial cells by the time of inflammation. Parallel to this, nuclear translocation of EGR1 was shown by immunocytochemistry at the day 5 of inflammation. Caveolin-1 level was high and ERK1/2 became phosphorylated as the inflammation proceeded showing a slight decrease when the regeneration started. Our present data support the idea that under special stimuli, mesenteric mesothelial cells are able to transdifferentiate into macrophages, and this transition is regulated by the caveolin-1/ERK1/2/EGR1 signaling pathway.





Similar content being viewed by others
References
van Furth, R., Z.A. Cohn, J.G. Hirsch, J.H. Humphry, W.G. Spector, and H.L. Lange-Woort. 1972. Mononuclear phagocytic system: New classification of macrophages, monocytes and their cell line. Bulletin of the World Health Organization 47: 651–658.
Van Furth, R. 1988. Phagocytic cells: Development and distribution of mononuclear phagocytes in normal steady state and inflammation. In Inflammation. Basic principles and clinical correlates, ed. J.I. Gallin, I.M. Goldstein, and R. Snyderman, 281–295. New York: Raven Press.
Ginsel, L.A., L.P. Rijfkogel, and W.T. Deams. 1985. A dual origin of macrophages? Review and hypothesis. In Macrophage biology, ed. S. Reichard and M. Kojima, 621–649. New York Press.
De Bakker, J.M., A.W. de Wit, H. Woelders, L.A. Ginsel, and W.T. Deams. 1985. On the origin of peritoneal resident macrophages. II. Recovery of the resident macrophage population in the peritoneal cavity and milky spots after peritoneal cell depletion. Journal of Submicroscopic Cytology 7: 141–151.
Papadimitriou, J.M., and R.B. Ashman. 1989. Macrophages: Current view on their differentiation, structure and function. Ultrastructural Pathology 13: 343–372.
Kiss, A.L., and A. Kittel. 1995. Early endocytotic steps in elicited macrophages: Omega-shaped plasma membrane vesicles at their cell surface. Cell Biology International 9: 527–538.
Geissmann, F., M.G. Manz, S. Jung, M.H. Sieweke, M. Merad, and K. Ley. 2010. Development of monocytes, macrophages and dendritic cells. Science 327: 656–661.
Katz, S., P. Balogh, and A.L. Kiss. 2011. Mesothelial cells can detach from the mesentery and differentiate into macrophage-like cells. Acta Pathologica, Microbiologica, et Immunologica Scandinavica 119: 782–793.
Katz, S., P. Balogh, N. Nagy, and A.L. Kiss. 2012. Epithelial-to-mesenchymal transition induced by Freund’s adjuvant treatment in rat mesothelial cells: A morphological and immunocytochemical study. Pathology Oncology Research 18: 641–649.
Katz, S., V. Zsiros, N. Doczi, A. Szabó, Á. Biczó, and A.L. Kiss. 2016. GM-CSF and GM-CSF receptor have regulatory role in transforming rat mesenteric mesothelial cells into macrophage-like cells. Inflammation Research 65: 827–836.
Wiese, C., A. Rolletschek, G. Kania, P. Blyszczuk, K.V. Tarasova, R.P. Wersto, K.R. Boheler, and A.M. Wobus. 2004. Nestin expression-a property of multi-lineage progenitor cells? Cellular and Molecular Life Sciences 61: 2510–2522.
Parameswaran, N., and S. Patial. 2010. Tumor necrosis factor-α signaling in macrophages. Critical Reviews in Eukaryotic Gene Expression 20 (2): 87–103.
Laslo, P., C.J. Spooner, A. Warmflash, D.W. Laneki, H.J. Lee, R. Sciammas, B.N. Gantner, A.R. Dinner, and H. Singh. 2006. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell 126: 755–766.
Krishnaraju, K., H.Q. Nguyen, D.A. Liebermann, and B. Hoffman. 1995. The zinc finger transcription factor Egr-1 potentiates macrophage differentiation of hematopoietic cells. Molecular and Cellular Biology 15: 5499–5507.
Krishnaraju, K., B. Hoffman, and D.A. Liebermann. 1998. The zinc finger transcription factor EGR-1 activates macrophage differentiation in M1 myeloblastic leukemia cells. Blood 92: 1957–1966.
Krishnaraju, K., B. Hoffman, and D.A. Liebermann. 2001. Early growth response gene 1 stimulates development of hematopoietic progenitor cells along the macrophage lineage at the expense of the granulocyte and erythroid lineages. Blood 97: 1298–1305.
Nguyen, H.Q.B., B. Hoffman-Liebermann, and D.A. Liebermann. 1993. The zinc finger transcription factor EGR-1 is essential for and restricts differentiation along macrophage lineage. Cell 72: 197–209.
Carter, J.H., and W.G. Tourtellotte. 2007. Early response transcriptional regulators are dispensable for macrophage differentiation. Journal of Immunology 178: 3038–3047.
Baron, V., E.D. Adamson, A. Calogero, G.F. Ragona, and D. Mercola. 2006. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53 and fibronectin. Cancer Gene Therapy 13: 115–124.
Fu, Y., X.-L. Moore, M.K.S. Lee, M.A. Fernandez-Rojo, M.-O. Parat, R.G. Parton, P.J. Meikle, D. Sviridov, and J.P. Chin-Dusting. 2012. Caveolin-1 plays a critical role in the differentiation of monocytes into macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology 32: 117–125.
Hume, D.A., H. Ross, S.R. Himes, R.T. Sasmono, C.A. Well, and T. Ravasi. 2002. The mononuclear phagocyíte system revisited. Journal of Leukocyte Biology 72: 621–627.
Slot, J.W., and H.J. Geuze. 2007. Cryosectioning and immunolabeling. Nature Protocols 2: 2480–2491.
Olson, B.J., and J. Markwell. 2007. Assays for determination of protein concentration. Current Protocols in Protein Science. https://doi.org/10.1002/0471140864.ps0304s48.
Rasko, J.E.J., and M.M. Grough. 1994. Granulocyte macrophage-colony stimulating factor. In Cytokine handbook, ed. A.W. Thomson, 2nd ed., 342–369. New York: Academic Press.
Bhattacharyya, S., S.J. Chen, M. Wu, M. Warner-Blankenship, H. Ning, G. Lakos, Y. Mori, E. Chang, C. Nihijima, K. Takehara, C. Feghali-Bostwick, and J. Varga. 2008. Smad-independent transforming growth factor-beta regulation of early growth response-1 and sustained expression in fibrosis: Implications for scleroderma. The American Journal of Pathology 173: 1085–1099.
Balogh, P., A. Szabó, S. Katz, I. Likó, A. Patócs, and A.L. Kiss. 2013. Estrogen receptor alpha is expressed in mesenteric mesothelial cells and is internalized in caveolae upon Freund’s adjuvant treatment. PLoS One 8: 1–10.
Anderson, R.G. 1993. Caveolae: Where incoming and outgoing messengers meet. Proceedings of the National Academy of Sciences of the United States of America 90: 10909–10913.
Scherer, P.E., Z. Tang, M. Chun, M. Sargiacomo, H.E. Lodish, and M.P. Lisanti. 1995. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. The Journal of Biological Chemistry 270: 16395–16401.
Fujimoto, T., H. Kogo, R. Nomura, and T. Une. 2000. Isoforms of caveolin-1 and caveolar structure. Journal of Cell Science 113: 3509–3517.
Nohe, A., E. Keating, C. Loh, T.M. Underhill, and N.O. Petersen. 2004. Caveolin-1 isoforms reorganization studied by image correlation spetroscopy. Faraday Discussions 126: 185–195.
Acknowledgements
We would like to express our thankfulness to Professor Pál Röhlich for the precious comments and accurate language correction of the manuscript. Special thanks are due to Katalin Lőcsey for her valuable technical help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Katz, S., Zsiros, V., Dóczi, N. et al. Inflammation-Induced Epithelial-to-Mesenchymal Transition and GM-CSF Treatment Stimulate Mesenteric Mesothelial Cells to Transdifferentiate into Macrophages. Inflammation 41, 1825–1834 (2018). https://doi.org/10.1007/s10753-018-0825-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0825-4