Abstract
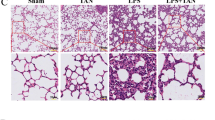
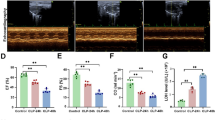
Sepsis-induced myocardial injury is a well-known cause of mortality. The cholinergic anti-inflammatory pathway (CHAIP) is a physiological mechanism by which the central nervous system regulates immune response through the vagus nerve and acetylcholine; the α7-nicotinic acetylcholine receptor (α7nAChR) is the main component of CHAIP; GTS-21, a synthetic α7nAChR selective agonist, has repeatedly shown its powerful anti-inflammatory effect. However, little is known about its effect on LPS-induced myocardial injury. We investigated the protective effects of GTS-21 on lipopolysaccharide (LPS)-induced cardiomyopathy via the cholinergic anti-inflammatory pathway in a mouse sepsis model. We constructed the model of myocardial injury in sepsis mice by C57BL/6 using LPS and determined the time of LPS treatment by hematoxylin-eosin (HE) and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL). C57BL/6 mice were randomized into five groups: blank control group, model group, α-bungarotoxin + LPS group, GTS-21 + LPS group, and α-bungarotoxin + GTS-21 + LPS group. The pathological results of myocardial tissue were detected by the HE method; the apoptosis rate was detected by the TUNEL method; the relative expressions of NF-κB p65, Caspase-3, Caspase-8, Bcl-2, Bax, p53, and a7nAChR were detected by real-time quantitative PCR (RT-PCR); and the protein expressions of IL-6, IL-1 β, TNF-α, and pSTAT3 were detected by western blot. The results showed that LPS-induced myocardial pathological and apoptosis changes were significant compared with the blank group, which was reversed by GTS-21; however, pretreatment with α-bungarotoxin obviously blocked the protective effect of GTS-21. NF-κB p65, Caspase-3, Caspase-8, Bax, p53, IL-6, IL-1β, TNF-α, and pSTAT3 were significantly increased in the model group, while a7nAChR and Bcl-2 were significantly decreased; GTS-21 treatment reversed that result, while pretreatment with α-bungarotoxin strengthened the result in the model. And pretreatment with α-bungarotoxin blocked the protective effect of GTS-21. GTS-21 can alleviate the LPS-induced damage in the heart via a7nAChR, and pretreatment with α-bungarotoxin obviously blocked the protective effect of GTS-21 on sepsis in mice.






Similar content being viewed by others
References
Fleischmann, C., A. Scherag, N.K. Adhikari, C.S. Hartog, T. Tsaganos, P. Schlattmann, D.C. Angus, and K. Reinhart. 2016. Assessment of global incidence and mortality of hospital-treated sepsis—current estimates and limitations. American Journal of Respiratory & Critical Care Medicine 193 (3): 259.
Weber, G.F., B.G. Chousterman, S. He, A.M. Fenn, M. Nairz, A. Anzai, T. Brenner, F. Uhle, Y. Iwamoto, and C.S. Robbins. 2015. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 347 (6227): 1260–1265.
Liu, A., W. Wang, H. Fang, Y. Yang, X. Jiang, S. Liu, J. Hu, Q. Hu, U. Dahmen, and O. Dirsch. 2015. Baicalein protects against polymicrobial sepsis-induced liver injury via inhibition of inflammation and apoptosis in mice. European Journal of Pharmacology 748: 45–53.
Petronilho, F., S.R. Périco, F. Vuolo, F. Mina, L. Constantino, C.M. Comim, J. Quevedo, D.O. Souza, and F. Dalpizzol. 2012. Protective effects of guanosine against sepsis-induced damage in rat brain and cognitive impairment. Brain Behavior & Immunity 26 (6): 904.
Silva, P.L., F.F. Cruz, L.C. Fujisaki, G.P. Oliveira, C.S. Samary, D.S. Ornellas, T. Marongutierrez, N.N. Rocha, R. Goldenberg, and C.S. Garcia. 2010. Hypervolemia induces and potentiates lung damage after recruitment maneuver in a model of sepsis-induced acute lung injury. Critical Care 14 (3): R114.
Sordi, R., D. Fernandes, B.T. Heckert, and J. Assreuy. 2011. Early potassium channel blockade improves sepsis-induced organ damage and cardiovascular dysfunction. British Journal of Pharmacology 163 (6): 1289–1301.
Fattahi, F., M. Kalbitz, E.A. Malan, E. Abe, L. Jajou, M.S. Huber-Lang, M. Bosmann, M.W. Russell, F.S. Zetoune, and P.A. Ward. 2017. Complement-induced activation of MAPKs and Akt during sepsis: role in cardiac dysfunction. Faseb Journal Official Publication of the Federation of American Societies for Experimental Biology 31 (9): 4129–4139.
Dal-Secco, D., S. DalBó, N.E.S. Lautherbach, F.N. Gava, M.R.N. Celes, P.O. Benedet, A.H. Souza, J. Akinaga, V. Lima, K.P. Silva, et al. 2017. Cardiac hyporesponsiveness in severe sepsis is associated with nitric oxide-dependent activation of G-protein receptor kinase. American Journal of Physiology - Heart and Circulatory Physiology 313 (1): H149–H163.
Feng, H., J. Chen, H. Wang, Y. Cheng, Z. Zou, Q. Zhong, and J. Xu. 2017. Roflumilast reverses polymicrobial sepsis-induced liver damage by inhibiting inflammation in mice. Laboratory Investigation 97 (9): 1008–1019.
Yu, C., P. Li, D. Qi, L. Wang, H.L. Qu, Y.J. Zhang, X.K. Wang, and H.Y. Fan. 2017. Osthole protects sepsis-induced acute kidney injury via down-regulating NF-κB signal pathway. Oncotarget 8 (3): 4796–4813.
Baghel, K., R.N. Srivastava, A. Chandra, S.K. Goel, J. Agrawal, H.R. Kazmi, and S. Raj. 2014. TNF-α, IL-6, and IL-8 cytokines and their association with TNF-α-308 G/a polymorphism and postoperative sepsis. Journal of Gastrointestinal Surgery 18 (8): 1486–1494.
Wu, H., J. Liu, W. Li, G. Liu, and Z. Li. 2016. LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of LPS-induced sepsis mice by activating NF-κB pathway. Biochemical & Biophysical Research Communications 471 (1): 240–246.
Duris, K., J. Lipkova, and M. Jurajda. 2017. Cholinergic anti-inflammatory pathway and stroke. Current Drug Delivery 14 (4): 449–457.
Treinin, M., R.L. Papke, E. Nizri, Y. Ben-David, T. Mizrachi, and T. Brenner. 2016. Role of the α7 nicotinic acetylcholine receptor and RIC-3 in the cholinergic anti-inflammatory pathway. Central Nervous System Agents in Medicinal Chemistry 16 (999): 1–9.
Altavilla, D., S. Guarini, A. Bitto, C. Mioni, D. Giuliani, A. Bigiani, G. Squadrito, L. Minutoli, F.S. Venuti, and F. Messineo. 2006. Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock 25 (5): 500–506.
Hoover, D.B. 2017. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacology & Therapeutics 179: 1–16.
Bonaz, B., V. Sinniger, and S. Pellissier. 2016. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. Journal of Physiology 594 (20): 5781.
Li, S., B. Zhou, B. Liu, Y. Zhou, H. Zhang, T. Li, and X. Zuo. 2016. Activation of the cholinergic anti-inflammatory system by nicotine attenuates arthritis via suppression of macrophage migration. Molecular Medicine Reports 14 (6):5057–5064.
Bonaz, B., V. Sinniger, and S. Pellissier. 2016. Vagal tone: effects on sensitivity, motility, and inflammation. Neurogastroenterology & Motility the Official Journal of the European Gastrointestinal Motility Society 28 (4): 455–462.
Zhang, Rong, N. Wugeti, Juan Sun, Huang Yan, Yujun Guo, Ling Zhang, Mei Ma, Xingui Guo, Changan Jiao, Wenli Xu, Tianqi Li, Haili Liu, and Yitong Ma. 2014. Effects of vagus nerve stimulation via cholinergic anti-inflammatory pathway activation on myocardial ischemia/reperfusion injury in canine. International Journal of Clinical & Experimental Medicine 7 (9): 2615–2623.
Shinlapawittayatorn, K., K. Chinda, S. Palee, S. Surinkaew, K. Thunsiri, P. Weerateerangkul, S. Chattipakorn, B.H. Kenknight, and N. Chattipakorn. 2013. Low-amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia-reperfusion injury. Heart Rhythm 10 (11): 1700–1707.
Endo, M., M. Hori, H. Ozaki, T. Oikawa, and T. Hanawa. 2014. Daikenchuto, a traditional Japanese herbal medicine, ameliorates postoperative ileus by anti-inflammatory action through nicotinic acetylcholine receptors. Journal of Gastroenterology 49 (6): 1026–1039.
Li, Y., and X. Shi. 2013. Anti-inflammatory effects of Kupffer cells through α7-nicotinic acetylcholine receptors. Critical Care 17 (Suppl 2): P7–P7.
Lisha, G., C. Xingxing, W. Lianpin, Z. Depu, L. Xiaowei, L. Jiafeng, and L. Yuechun. 2017. Right cervical vagotomy aggravates viral myocarditis in mice via the cholinergic anti-inflammatory pathway. Frontiers in Pharmacology 8: 25.
Khan, M.A., M. Farkhondeh, J. Crombie, L. Jacobson, M. Kaneki, and J.A. Martyn. 2012. Lipopolysaccharide upregulates α7 acetylcholine receptors: stimulation with GTS-21 mitigates growth arrest of macrophages and improves survival in burned mice. Shock 38 (2): 213.
Kashiwagi, S., M.A. Khan, S. Yasuhara, T. Goto, W.R. Kem, R.G. Tompkins, M. Kaneki, and J.A. Martyn. 2017. Prevention of burn-induced inflammatory responses and muscle wasting by GTS-21, a specific agonist for α7 nicotinic acetylcholine receptors. Shock 47 (1): 61.
Pavlov, Valentin A., Mahendar Ochani, LiHong Yang, Margot Gallowitsch-Puerta, and Kanta Ochani. 2007. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis*. Critical Care Medicine 35 (35): 1139–1144.
Yue, Y., R. Liu, W. Cheng, Y. Hu, J. Li, X. Pan, J. Peng, and P. Zhang. 2015. GTS-21 attenuates lipopolysaccharide-induced inflammatory cytokine production in vitro by modulating the Akt and NF-κB signaling pathway through the α7 nicotinic acetylcholine receptor. International Immunopharmacology 29 (2): 504.
daCosta, C.J.B., C.R. Free, and S.M. Sine. 2015. Stoichiometry for α-bungarotoxin block of α7 acetylcholine receptors. Nat Commun 6: 8057.
Kempsill, F.E., P.J. Covernton, P.J. Whiting, and J.G. Connolly. 1999. Agonist activation and alpha-bungarotoxin inhibition of wild type and mutant alpha7 nicotinic acetylcholine receptors. European Journal of Pharmacology 383 (3): 347–359.
Wu, S., H. Zhao, H. Luo, X. Xiao, H. Zhang, T. Li, and X. Zuo. 2014. GTS-21, an α7-nicotinic acetylcholine receptor agonist, modulates Th1 differentiation in CD4(+) T cells from patients with rheumatoid arthritis. Experimental & Therapeutic Medicine 8 (2): 557–562.
Xianchu, L., P.Z. Lan, L. Qiufang, L. Yi, R. Xiangcheng, H. Wenqi, and D. Yang. 2016. Naringin protects against lipopolysaccharide-induced cardiac injury in mice. Environmental Toxicology and Pharmacology 48: 1–6.
Niu, J., K. Wang, S. Graham, A. Azfer, and P.E. Kolattukudy. 2011. MCP-1-induced protein attenuates endotoxin-induced myocardial dysfunction by suppressing cardiac NF-кB activation via inhibition of IкB kinase activation. Journal of Molecular and Cellular Cardiology 51 (2): 177–186.
Wang, Z., Q. Wu, X. Nie, J. Guo, and C. Yang. 2015. Infusion of esmolol attenuates LPS-induced myocardial dysfunction. J Surg Res 200 (1): 283–289.
Jung, J.Y., Y.H. Kwak, I. Chang, W.Y. Kwon, G.J. Suh, and D. Choi. 2017. Protective effect of hemopexin on systemic inflammation and acute lung injury in an endotoxemia model. Journal of Surgical Research 212: 15.
Genga, K.R., and J.A. Russell. 2017. Update of sepsis in the intensive care unit. Innate Immunity 9 (5): 441–455.
Hotchkiss, R.S., and I.E. Karl. 2003. The pathophysiology and treatment of sepsis. New England Journal of Medicine 348 (2): 138.
Dal-Secco, D., S. Dalbó, L. Nes, F.N. Gava, C. Mrn, P.O. Benedet, A.H. Souza, J. Akinaga, V. Lima, and K.P. Silva. 2017. Cardiac hyporesponsiveness in severe sepsis is associated with nitric oxide-dependent activation of G-protein receptor kinase. Ajp Heart & Circulatory Physiology. https://doi.org/10.1152/ajpheart.00052.2016.
Zanotticavazzoni, S.L., and S.M. Hollenberg. 2009. Cardiac dysfunction in severe sepsis and septic shock. Current Opinion in Critical Care 15 (5): 392.
Nevière, R., H. Fauvel, C. Chopin, P. Formstecher, and P. Marchetti. 2001. Caspase inhibition prevents cardiac dysfunction and heart apoptosis in a rat model of sepsis. American Journal of Respiratory & Critical Care Medicine 163 (1): 218.
Zaky, A., S. Deem, K. Bendjelid, and M.M. Treggiari. 2014. Characterization of cardiac dysfunction in sepsis: an ongoing challenge. Shock 41 (1): 12–24.
Rossi, M.A., M.R. Celes, C.M. Prado, and F.P. Saggioro. 2007. Myocardial structural changes in long-term human severe sepsis/septic shock may be responsible for cardiac dysfunction. Heart Lung & Circulation 27 (1): 10.
Tan, Y., Q. Wang, Y. She, X. Bi, and B. Zhao. 2015. Ketamine reduces LPS-induced HMGB1 via activation of the Nrf2/HO-1 pathway and NF-κB suppression. Journal of Trauma & Acute Care Surgery 78 (4): 784–792.
S S, D.L., Y.X. M B, L.W. W X, and Z.Q. Z W. 2016. Gx-50 inhibits neuroinflammation via α7 nAChR activation of the JAK2/STAT3 and PI3K/AKT pathways. Journal of Alzheimer's Disease 50 (3): 859–871.
Kox, M., J.C. Pompe, M.C. Gordinou de Gouberville, J.G. van der Hoeven, C.W. Hoedemaekers, and P. Pickkers. 2009. Effects of the alpha7nAChR Agonist GTS-21 on the Innate Immune Response in Humans. Shock 36 (1): 5–11.
Funding
This project was funded by the National Natural Science Foundation of China (No.81571871), the National Natural Science Foundation of China (No.81770276 ), the National Natural Science Foundation of China(No.81772045), Nn10 program of Harbin Medical University Cancer Hospital, Heilongjiang Health Department project (No.2017-001), Postdoctoral Funding of Heilongjiang Province (No. LBH-Z16147), and Talent Fund of Harbin Science and Technology Bureau (No.2017RAQXJ177).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experiments conformed to guidelines established by the Ministry of Health, PR China, and approved by the animal care committee of Harbin Medical University (Harbin, China).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Kai Kang Co-first author
Rights and permissions
About this article
Cite this article
Kong, W., Kang, K., Gao, Y. et al. GTS-21 Protected Against LPS-Induced Sepsis Myocardial Injury in Mice Through α7nAChR. Inflammation 41, 1073–1083 (2018). https://doi.org/10.1007/s10753-018-0759-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0759-x