Abstract
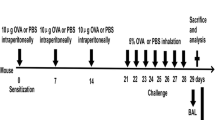
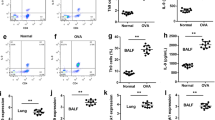
Interaction between T cells and airway smooth muscle (ASM) cells has been identified as an important factor in the development of asthma. LIGHT (known as TNFSF14) -mediated signaling likely contributes to various inflammatory disorders and airway remodeling. The objective of this study was to investigate the roles of LIGHT-mediated pathways in the interaction between ASM cells and T cells during chronic allergic inflammation. Mice were sensitized and challenged by ovalbumin (OVA) to induce chronic airway allergic inflammation. The control group received PBS only. The histological features and LIGHT expressions in lungs were assessed in vivo. Furthermore, T cells and ASM cells derived from the model mice were co-cultured both in the presence and absence of anti-LIGHT Ab for 72 h. The effects of LIGHT blockade on expressions of downstream signaling molecules, proliferation, and apoptosis of ASM cells, differentiation of T cells, and inflammatory cytokines release were evaluated. We demonstrated that LIGHT blockade strikingly inhibited the mRNA and protein expressions of HVEM, c-JUN, and NFκB. Additionally, LIGHT blockade resulted in decreased proliferation and increased apoptosis of ASM cells. Moreover, depletion of LIGHT dramatically reduced the differentiation of CD4+ T cells into Th1, Th2, and Th17 cells, as well as inhibited inflammatory cytokines release including IL-13, TGF-β, and IFN-γ, which are associated with CD4+ T cell differentiation and ASM cell proliferation. LIGHT plays an important role in the interaction between T cells and ASM cells in chronic allergic asthma. Blockade of LIGHT markedly suppressed ASM hyperplasia and inflammatory responses, which might be modulated through HVEM-NFκB or c-JUN pathways. Therefore, targeting LIGHT is a promising therapeutic strategy for airway inflammation and remodeling in chronic allergic asthma.





Similar content being viewed by others
References
Tanabe, T., and B.K. Rubin. 2016. Airway goblet cells secrete pro-inflammatory cytokines, chemokines, and growth factors. Chest 149 (3): 714–720.
Bousquet, J., P.K. Jeffery, W.W. Busse, M. Johnson, and A.M. Vignola. 2000. Asthma. From bronchoconstriction to airways inflammation and remodeling. American Journal of Respiratory and Critical Care Medicine 161 (5): 1720–1745.
Khan, M.A., A.M. Assiri, and D.C. Broering. 2015. Complement mediators: Key regulators of airway tissue remodeling in asthma. Journal of Translational Medicine 13: 272.
Vieira, R.P., R.C. Claudino, A.C. Duarte, A.B. Santos, A. Perini, H.C. Faria Neto, T. Mauad, M.A. Martins, M. Dolhnikoff, and C.R. Carvalho. 2007. Aerobic exercise decreases chronic allergic lung inflammation and airway remodeling in mice. American Journal of Respiratory and Critical Care Medicine 176 (9): 871–877.
Kita, T., M. Fujimura, S. Myou, K. Watanabe, Y. Waseda, and S. Nakao. 2009. Effects of KF19514, a phosphodiesterase 4 and 1 inhibitor, on bronchial inflammation and remodeling in a murine model of chronic asthma. Allergology international : official journal of the Japanese Society of Allergology 58 (2): 267–275.
Shi, F., C. Qiu, H. Qi, and W. Peng. 2012. shRNA targeting beta1-integrin suppressed proliferative aspects and migratory properties of airway smooth muscle cells. Molecular and Cellular Biochemistry 361 (1–2): 111–121.
Fabry, Z., M.M. Waldschmidt, L. Van Dyk, S.A. Moore, and M.N. Hart. 1990. Activation of CD4+ lymphocytes by syngeneic brain microvascular smooth muscle cells. Journal of Immunology 145 (4): 1099–1104.
Hakonarson, H., C. Kim, R. Whelan, D. Campbell, and M.M. Grunstein. 2001. Bi-directional activation between human airway smooth muscle cells and T lymphocytes: Role in induction of altered airway responsiveness. Journal of Immunology 166 (1): 293–303.
Li, H., C. He, J. Feng, Y. Zhang, Q. Tang, Z. Bian, X. Bai, H. Zhou, H. Jiang, S.P. Heximer, et al. 2010. Regulator of G protein signaling 5 protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Proceedings of the National Academy of Sciences of the United States of America 107 (31): 13818–13823.
Kuo, P.L., Y.L. Hsu, M.J. Tsai, C.T. Lien, M.S. Huang, and Y.C. Ko. 2012. Nonylphenol induces bronchial epithelial apoptosis via Fas-mediated pathway and stimulates bronchial epithelium to secrete IL-6 and IL-8, causing bronchial smooth muscle proliferation and migration. Basic & Clinical Pharmacology & Toxicology 110 (2): 178–186.
Bechill, J., and W.J. Muller. 2014. Herpesvirus entry mediator (HVEM) attenuates signals mediated by the lymphotoxin beta receptor (LTbetaR) in human cells stimulated by the shared ligand LIGHT. Molecular Immunology 62 (1): 96–103.
Waldemer-Streyer, R.J., and J. Chen. 2015. Myocyte-derived Tnfsf14 is a survival factor necessary for myoblast differentiation and skeletal muscle regeneration. Cell Death & Disease 6: e2026.
Castellano, R., C. Van Lint, V. Peri, E. Veithen, Y. Morel, R. Costello, D. Olive, and Y. Collette. 2002. Mechanisms regulating expression of the tumor necrosis factor-related light gene. Role of calcium-signaling pathway in the transcriptional control. J Biol Chem 277 (45): 42841–42851.
Cheung, T.C. 2009. Modulation of T cell proliferation through the LIGHT-HVEM-BTLA cosignaling pathway. Recent Patents on DNA & Gene Sequences 3 (3): 177–182.
Doherty, T.A., P. Soroosh, N. Khorram, S. Fukuyama, P. Rosenthal, J.Y. Cho, P.S. Norris, H. Choi, S. Scheu, K. Pfeffer, et al. 2011. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nature Medicine 17 (5): 596–603.
Plant, P.J., M.L. North, A. Ward, M. Ward, N. Khanna, J. Correa, J.A. Scott, and J. Batt. 2012. Hypertrophic airway smooth muscle mass correlates with increased airway responsiveness in a murine model of asthma. American Journal of Respiratory Cell and Molecular Biology 46 (4): 532–540.
Scheu, S., J. Alferink, T. Potzel, W. Barchet, U. Kalinke, and K. Pfeffer. 2002. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. The Journal of Experimental Medicine 195 (12): 1613–1624.
Wang, J., J.C. Lo, A. Foster, P. Yu, H.M. Chen, Y. Wang, K. Tamada, L. Chen, and Y.X. Fu. 2001. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. The Journal of Clinical Investigation 108 (12): 1771–1780.
Wynn, T.A., and T.R. Ramalingam. 2011. Shedding LIGHT on severe asthma. Nature Medicine 17 (5): 547–548.
Shifren, A., C. Witt, C. Christie, and M. Castro. 2012. Mechanisms of remodeling in asthmatic airways. Journal of allergy 2012: 316049.
Dekkers, B.G., H. Maarsingh, H. Meurs, and R. Gosens. 2009. Airway structural components drive airway smooth muscle remodeling in asthma. Proceedings of the American Thoracic Society 6 (8): 683–692.
Renauld, J.C. 2001. New insights into the role of cytokines in asthma. Journal of Clinical Pathology 54 (8): 577–589.
Murdoch, J.R., and C.M. Lloyd. 2010. Chronic inflammation and asthma. Mutation Research 690 (1–2): 24–39.
Durrant, D.M., and D.W. Metzger. 2010. Emerging roles of T helper subsets in the pathogenesis of asthma. Immunological Investigations 39 (4–5): 526–549.
Kolls, J.K. 2013. CD4(+) T-cell subsets and host defense in the lung. Immunological Reviews 252 (1): 156–163.
Alvira, C.M. 2014. Nuclear factor-kappa-B signaling in lung development and disease: One pathway, numerous functions. Birth defects research Part A, Clinical and molecular teratology 100 (3): 202–216.
Bennett, B.L. 2006. c-Jun N-terminal kinase-dependent mechanisms in respiratory disease. The European Respiratory Journal 28 (3): 651–661.
Harrop, J.A., M. Reddy, K. Dede, M. Brigham-Burke, S. Lyn, K.B. Tan, C. Silverman, C. Eichman, R. DiPrinzio, J. Spampanato, et al. 1998. Antibodies to TR2 (herpesvirus entry mediator), a new member of the TNF receptor superfamily, block T cell proliferation, expression of activation markers, and production of cytokines. Journal of Immunology 161 (4): 1786–1794.
Pierer, M., F. Brentano, J. Rethage, U. Wagner, H. Hantzschel, R.E. Gay, S. Gay, and D. Kyburz. 2007. The TNF superfamily member LIGHT contributes to survival and activation of synovial fibroblasts in rheumatoid arthritis. Rheumatology (Oxford) 46 (7): 1063–1070.
Acknowledgements
We thank Jinan University and Shenzhen-PKU-HKUST Medical Center for the use of resources and facilities.
Funding
The study was supported by the National Natural Science Foundation of China (Project Number: 81300012), the Science and Technology Planning Project of Guangdong Province, China (Project Number: 2016A020215025), the Medical Scientific Research Foundation of Guangdong Province, China (Project Number: A2016375), and the Shenzhen Science and Technology Innovation Committee Basic Science Research Grant (JCYJ20140416144209745, JCYJ20160427185306518).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, F., Xiong, Y., Zhang, Y. et al. The Role of TNF Family Molecules Light in Cellular Interaction Between Airway Smooth Muscle Cells and T Cells During Chronic Allergic Inflammation. Inflammation 41, 1021–1031 (2018). https://doi.org/10.1007/s10753-018-0755-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0755-1