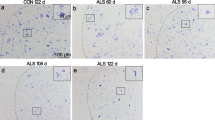
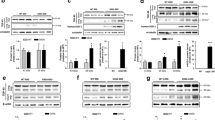
Abstract
Amyotrophic lateral sclerosis (ALS) is a disabling progressive disease characterized by the degeneration of motor neurons, leading to muscle atrophy and paralysis. The majority of cases are sporadic, but also a familiar form of ALS exists, and some genes causative of the pathology were found. In particular, mutations in superoxide dismutase 1 (SOD1) were found in 20% of familiar cases. It is known that neuroinflammation plays a pivotal role in several neurodegenerative disorders, including ALS. Inflammasomes are protein complexes that induce inflammation in response to various stimuli, involved also in neuroinflammation. The NLRP3 inflammasome, which is the best known, after assembly, induces the activation of caspase 1, which in turn activates interleukin (IL)-1β and IL-18. The aim of this work was the evaluation of inflammasome activation in the brain of SOD1G93A rats, a transgenic model of ALS. We observed the increase in TLR4 and nuclear NF-κB levels in SOD1G93A rats. Their activation is known as priming signal for inflammasome induction. Moreover, NLRP3 protein increased, associated with the presence of active caspase 1, leading to an increase in IL-18 and IL-1β levels. In addition, IL-1β, IL-18, and IFN-γ amount increased in the spleen of SOD1G93A rats, together with an increased expression of CD4, CD8, CD44, and CD68 markers. In conclusion, our results showed the activation of the NLRP3 inflammasome in the brain of SOD1G93A rats, indicating that inflammation plays a main role in ALS.






Similar content being viewed by others
References
Hardiman, Orla, Leonard H. van den Berg, and Matthew C. Kiernan. 2011. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nature Reviews Neurology 7 (11): 639–649.
Morgan, Sarah, and Richard W. Orrell. 2016. Pathogenesis of amyotrophic lateral sclerosis. British Medical Bulletin 119 (1): 87–98.
Byrne, Susan, Cathal Walsh, Catherine Lynch, Peter Bede, Marwa Elamin, Kevin Kenna, Russell McLaughlin, and Orla Hardiman. 2011. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. Journal of Neurology, Neurosurgery, and Psychiatry 82 (6): 623–627.
Zufiria, Monica, Francisco Javier Gil-Bea, Roberto Fernandez-Torron, Juan Jose Poza, Jose Luis Munoz-Blanco, Ricard Rojas-Garcia, Javier Riancho, and Adolfo Lopez de Munain. 2016. ALS: A bucket of genes, environment, metabolism and unknown ingredients. Progress in Neurobiology 142: 104–129.
Kaur, Simran J., Stephanie R. McKeown, and Shazia Rashid. 2016. Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene 577 (2): 109–118.
Philips, Thomas, and Jeffrey D. Rothstein. 2015. Rodent Models of Amyotrophic Lateral Sclerosis. Current protocols in pharmacology / editorial board, S J Enna (editor-in-chief) [et al ] 69:5.67.61–21.
Turner, Bradley J., and Kevin Talbot. 2008. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Progress in Neurobiology 85 (1): 94–134.
Hayashi, Yuki, Kengo Homma, and Hidenori Ichijo. 2016. SOD1 in neurotoxicity and its controversial roles in SOD1 mutation-negative ALS. Advances in biological regulation 60: 95–104.
Lee, J., S.J. Hyeon, H. Im, H. Ryu, Y. Kim, and H. Ryu. 2016. Astrocytes and Microglia as Non-cell Autonomous Players in the Pathogenesis of ALS. Experimental Neurology 25 (2): 233–240.
Chen, Wei-Wei, Xia Zhang, and Wen-Juan Huang. 2016. Role of neuroinflammation in neurodegenerative diseases (Review). Molecular Medicine Reports 13 (4): 3391–3396.
Philips, Thomas, and Wim Robberecht. 2011. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. The Lancet Neurology 10 (3): 253–263.
Komine, Okiru, and Koji Yamanaka. 2015. Neuroinflammation in motor neuron disease. Nagoya Journal of Medical Science 77 (4): 537–549.
Singhal, Gaurav, Emily J. Jaehne, Frances Corrigan, Catherine Toben, and Bernhard T. Baune. 2014. Inflammasomes in neuroinflammation and changes in brain function: a focused review. Frontiers in Neuroscience 8: 315.
Davis, Beckley K., Haitao Wen, and Jenny P.Y. Ting. 2011. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annual Review of Immunology 29: 707–735.
Zhou, Keren, Ligen Shi, Yan Wang, Sheng Chen, and Jianmin Zhang. 2016. Recent Advances of the NLRP3 Inflammasome in Central Nervous System Disorders. Journal of Immunology Research 2016: 9238290.
He, Y., H. Hara, and G. Núñez. 2016. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends in Biochemical Sciences 41 (12): 1012–1021. https://doi.org/10.1016/j.tibs.2016.09.002.
Turner, M.R., A. Cagnin, F.E. Turkheimer, C.C.J. Miller, C.E. Shaw, D.J. Brooks, P.N. Leigh, and R.B. Banati. 2004. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiology of Disease 15 (3): 601–609.
Corcia, Philippe, Clovis Tauber, Johnnie Vercoullie, Nicolas Arlicot, Caroline Prunier, Julien Praline, Guillaume Nicolas, et al. 2012. Molecular imaging of microglial activation in amyotrophic lateral sclerosis. PloS One 7 (12): e52941.
Brettschneider, Johannes, Jon B. Toledo, Vivianna M. Van Deerlin, Lauren Elman, Leo McCluskey, Virginia M.Y. Lee, and John Q. Trojanowski. 2012. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PloS One 7 (6): e39216.
Alexianu, M.E., M. Kozovska, and S.H. Appel. 2001. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology 57 (7): 1282–1289.
Martinon, Fabio, Kimberly Burns, and Jurg Tschopp. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular Cell 10 (2): 417–426.
Walsh, John G., Daniel A. Muruve, and Christopher Power. 2014. Inflammasomes in the CNS. Nature Reviews Neuroscience 15 (2): 84–97.
Gold, Maike, and Joseph El Khoury. 2015. Beta-amyloid, microglia, and the inflammasome in Alzheimer’s disease. Seminars in Immunopathology 37 (6): 607–611.
Zhang, Pei, Xiao-Yun Shao, Guang-Jian Qi, Qiang Chen, Lu-Lu Bu, Li-Jun Chen, Jing Shi, Jie Ming, and Bo Tian. 2016. Cdk5-Dependent Activation of Neuronal Inflammasomes in Parkinson’s Disease. Movement Disorders: Official Journal of the Movement Disorder Society 31 (3): 366–376.
Inoue, Makoto, and Mari L. Shinohara. 2013. NLRP3 Inflammasome and MS/EAE. Autoimmune diseases 2013: 859145.
Poltorak, A., P. Ricciardi-Castagnoli, S. Citterio, and B. Beutler. 2000. Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation. Proceedings of the National Academy of Sciences of the United States of America 97 (5): 2163–2167.
Trotta, Teresa, Chiara Porro, Rosa Calvello, and Maria Antonietta Panaro. 2014. Biological role of Toll-like receptor-4 in the brain. Journal of Neuroimmunology 268 (1–2): 1–12.
Olson, Julie K., and Stephen D. Miller. 2004. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. Journal of immunology (Baltimore, Md: 1950) 173 (6): 3916–3924.
Tang, Sung-Chun, Thiruma V. Arumugam, Xiangru Xu, Aiwu Cheng, Mohamed R. Mughal, Dong Gyu Jo, Justin D. Lathia, et al. 2007. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proceedings of the National Academy of Sciences of the United States of America 104 (34): 13798–13803.
De Paola, Massimiliano, Alessandro Mariani, Paolo Bigini, Marco Peviani, Giovanni Ferrara, Monica Molteni, Sabrina Gemma, et al. 2012. Neuroprotective effects of toll-like receptor 4 antagonism in spinal cord cultures and in a mouse model of motor neuron degeneration. Molecular medicine (Cambridge, Mass ) 18: 971–981.
Letiembre, Maryse, Liu Yang, Silke Walter, Wenlin Hao, Tatjana Pfander, Arne Wrede, Walter Schulz-Schaeffer, and Klaus Fassbender. 2009. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiology of Aging 30 (5): 759–768.
Casula, M., A.M. Iyer, W.G.M. Spliet, J.J. Anink, K. Steentjes, M. Sta, D. Troost, and E. Aronica. 2011. Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience 179: 233–243.
De Paola, Massimiliano, Stefania E. Sestito, Alessandro Mariani, Christian Memo, Roberto Fanelli, Mattia Freschi, Caterina Bendotti, Valentina Calabrese, and Francesco Peri. 2016. Synthetic and natural small molecule TLR4 antagonists inhibit motoneuron death in cultures from ALS mouse model. Pharmacological Research 103: 180–187.
Lee, Jia Y., John D. Lee, Simon Phipps, Peter G. Noakes, and Trent M. Woodruff. 2015. Absence of toll-like receptor 4 (TLR4) extends survival in the hSOD1 G93A mouse model of amyotrophic lateral sclerosis. Journal of Neuroinflammation 12: 90.
Lee, Myeong Sup, and Young-Joon Kim. 2007. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annual Review of Biochemistry 76: 447–480.
Frakes, Ashley E., Laura Ferraiuolo, Amanda M. Haidet-Phillips, Leah Schmelzer, Lyndsey Braun, Carlos J. Miranda, Katherine J. Ladner, et al. 2014. Microglia induce motor neuron death via the classical NF-kappaB pathway in amyotrophic lateral sclerosis. Neuron 81 (5): 1009–1023.
Li, M., V.O. Ona, C. Guegan, M. Chen, V. Jackson-Lewis, L.J. Andrews, A.J. Olszewski, et al. 2000. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science (New York, N Y ) 288 (5464): 335–339.
Pasinelli, P., M.K. Houseweart, R.H. Brown Jr., and D.W. Cleveland. 2000. Caspase-1 and -3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proceedings of the National Academy of Sciences of the United States of America 97 (25): 13901–13906.
Johann, Sonja, Marius Heitzer, Mithila Kanagaratnam, Anand Goswami, Tania Rizo, Joachim Weis, Dirk Troost, and Cordian Beyer. 2015. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 63 (12): 2260–2273.
Heitzer, M., S. Kaiser, M. Kanagaratnam, A. Zendedel, P. Hartmann, C. Beyer, and S. Johann. 2016. Administration of 17beta-Estradiol Improves Motoneuron Survival and Down-regulates Inflammasome Activation in Male SOD1(G93A) ALS Mice. Molecular Neurobiology. https://doi.org/10.1007/s12035-016-0322-4.
Debye, B., L. Schmülling, L. Zhou, G. Rune, C. Beyer, and S. Johann. 2016. Neurodegeneration and NLRP3 inflammasome expression in the anterior thalamus of SOD1(G93A) ALS mice. Brain Pathology. https://doi.org/10.1111/bpa.12467.
Italiani, Paola, Cecilia Carlesi, Paola Giungato, Ilaria Puxeddu, Barbara Borroni, Paola Bosso, Paola Migliorini, Gabriele Siciliano, and Diana Boraschi. 2014. Evaluating the levels of interleukin-1 family cytokines in sporadic amyotrophic lateral sclerosis. Journal of Neuroinflammation 11: 94.
Kadhim, Hazim, Paul Deltenre, Jean-Jacques Martin, and Guillaume Sebire. 2016. In-situ expression of Interleukin-18 and associated mediators in the human brain of sALS patients: Hypothesis for a role for immune-inflammatory mechanisms. Medical Hypotheses 86: 14–17.
Zhao, Weihua, David R. Beers, Shaughn Bell, Jinghong Wang, Shixiang Wen, Robert H. Baloh, and Stanley H. Appel. 2015. TDP-43 activates microglia through NF-kappaB and NLRP3 inflammasome. Experimental Neurology 273: 24–35.
Masters, Seth L., and Luke A.J. O'Neill. 2011. Disease-associated amyloid and misfolded protein aggregates activate the inflammasome. Trends in Molecular Medicine 17 (5): 276–282.
Kang, Jihong, and Serge Rivest. 2007. MyD88-deficient bone marrow cells accelerate onset and reduce survival in a mouse model of amyotrophic lateral sclerosis. The Journal of Cell Biology 179 (6): 1219–1230.
Meissner, Felix, Kaaweh Molawi, and Arturo Zychlinsky. 2010. Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proceedings of the National Academy of Sciences of the United States of America 107 (29): 13046–13050.
Rasouli, J., R. Lekhraj, M. Ozbalik, P. Lalezari, and D. Casper. 2011. Brain-Spleen Inflammatory Coupling: A Literature Review. The Einstein Journal of Biology and Medicine: EJBM 27 (2): 74–77.
Banerjee, Rebecca, R. Lee Mosley, Ashley D. Reynolds, Alok Dhar, Vernice Jackson-Lewis, Paul H. Gordon, Serge Przedborski, and Howard E. Gendelman. 2008. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PloS One 3 (7): e2740.
Okamura, H., H. Tsutsi, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, and K. Hattori. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378 (6552): 88–91.
Aebischer, J., P. Cassina, B. Otsmane, A. Moumen, D. Seilhean, V. Meininger, L. Barbeito, B. Pettmann, and C. Raoul. 2011. IFNgamma triggers a LIGHT-dependent selective death of motoneurons contributing to the non-cell-autonomous effects of mutant SOD1. Cell Death and Differentiation 18 (5): 754–768.
Garlanda, Cecilia, Charles A. Dinarello, and Alberto Mantovani. 2013. The interleukin-1 family: back to the future. Immunity 39 (6): 1003–1018.
Dinarello, Charles A. 2006. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. The American Journal of Clinical Nutrition 83 (2): 447S–455S.
Berg, Rance E., Christoph J. Cordes, and James Forman. 2002. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. European Journal of Immunology 32 (10): 2807–2816.
Acknowledgements
We thank Dr. Filippo Fraggetta from “Azienda Ospedaliera Cannizzaro” (Catania, Italy) for his contribution with immunohistochemical analysis.
Funding
This study was supported by current research fund 2017, Ministry of Health, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Gugliandolo, A., Giacoppo, S., Bramanti, P. et al. NLRP3 Inflammasome Activation in a Transgenic Amyotrophic Lateral Sclerosis Model. Inflammation 41, 93–103 (2018). https://doi.org/10.1007/s10753-017-0667-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0667-5