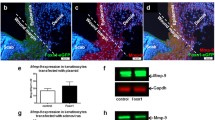
Abstract
Inflammatory cells exert crucial influence on wound healing, while exploration is still desired to get further insight into the key factors that promote the process. In the present study, we performed comparative microarray data analysis of the three types of inflammatory cells isolated from skin wounds and focused on differentially expressed secreted factors. Gene Ontology enrichment and receptor analysis indicated that 11 genes of secreted factors in Ly6C+ inflammatory macrophages and 27 genes of secreted factors in neutrophils exhibited higher (≥ 5-fold) expression, and all these factors were considered as candidates with potentially important role in keratinocyte activation. Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis revealed that TNF and IL17 signaling pathways were activated by Ly6C+ inflammatory macrophages secreted cytokines, while TGF-beta, HIF-1, NF-κB, and Rap 1 signaling pathways and pathways regulating pluripotency of stem cells were highly involved with neutrophils secreted cytokines. Taking TNF as an example, the source of the cytokine and its impact on keratinocytes were verified by immunofluorescence, gene knockout mice, and quantitative phosphoproteomics. Collectively, this study has indicated distinctively expressed cytokines in three inflammatory cells, which is helpful in identifying key factors in inducing keratinocyte signaling and in wound healing.




Similar content being viewed by others
References
Singer, A.J., and R.A. Clark. 1999. Cutaneous wound healing. The New England Journal of Medicine 341 (10): 738–746.
Martin, P. 1997. Wound healing—aiming for perfect skin regeneration. Science 276 (5309): 75–81.
Pastar, I., et al. 2014. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care (New Rochelle) 3 (7): 445–464.
Raja, et al. 2007. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Frontiers in Bioscience 12: 2249–2268.
Usui, M.L., et al. 2008. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. Journal of Histochemistry & Cytochemistry 56 (7): 687–696.
Pastar, I., O. Stojadinovic, and M. Tomic-Canic. 2008. Role of keratinocytes in healing of chronic wounds. Surgical Technology International 17: 105–112.
Theilgaard-Monch, K., et al. 2004. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. Journal of Immunology 172 (12): 7684–7693.
Tomic-Canic, M., et al. 1998. Epidermal signal transduction and transcription factor activation in activated keratinocytes. Journal of Dermatological Science 17 (3): 167–181.
Gordon, S. 2003. Alternative activation of macrophages. Nature Reviews. Immunology 3 (1): 23–35.
Gordon, S., and F.O. Martinez. 2010. Alternative activation of macrophages: mechanism and functions. Immunity 32 (5): 593–604.
Wang, X., et al. 2017. Macrophages induce AKT/β-catenin-dependent Lgr5+ stem cell activation and hair follicle regeneration through TNF. Nature Communications 8: 14091.
Dovi, J.V., A.M. Szpaderska, and L.A. DiPietro. 2004. Neutrophil function in the healing wound: adding insult to injury? Thrombosis and Haemostasis 92 (2): 275–280.
Gallucci, R.M., et al. 2004. Interleukin 6 indirectly induces keratinocyte migration. The Journal of Investigative Dermatology 122 (3): 764–772.
Lin, Z.Q., et al. 2003. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. Journal of Leukocyte Biology 73 (6): 713–721.
Ishida, Y., et al. 2004. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. Journal of Immunology 172 (3): 1848–1855.
Gillitzer, R., and M. Goebeler. 2001. Chemokines in cutaneous wound healing. Journal of Leukocyte Biology 69 (4): 513–521.
Zaja-Milatovic, S., and A. Richmond. 2008. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histology and Histopathology 23 (11): 1399–1407.
Lawrence, W.T., and R.F. Diegelmann. 1994. Growth-Factors in Wound-Healing. Clinics in Dermatology 12 (1): 157–169.
Grotendorst, G.R., et al. 1989. Egf and Tgf-Alpha Are Potent Chemoattractants for Endothelial-Cells and Egf-Like Peptides Are Present at Sites of Tissue Regeneration. Journal of Cellular Physiology 139 (3): 617–623.
Mirza, R., L.A. DiPietro, and T.J. Koh. 2009. Selective and Specific Macrophage Ablation Is Detrimental to Wound Healing in Mice. American Journal of Pathology 175 (6): 2454–2462.
Goren, I., et al. 2010. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic and contractive processes. Naunyn-Schmiedebergs Archives of Pharmacology 381: 37–37.
Davies, L.C., et al. 2013. Tissue-resident macrophages. Nature Immunology 14 (10): 986–995.
Shi, C., and E.G. Pamer. 2011. Monocyte recruitment during infection and inflammation. Nature Reviews. Immunology 11 (11): 762–774.
Wang, X.S., et al. 2013. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nature Protocols 8 (2): 302–309.
Wu, Y.J., et al. 2007. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25 (10): 2648–2659.
Bickenbach, J.R. 2005. Isolation, characterization, and culture of epithelial stem cells. Methods in Molecular Biology 289: 97–102.
Silva-Vargas, V., et al. 2005. beta-catenin and hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Developmental Cell 9 (1): 121–131.
Witte, M.B., and A. Barbul. 1997. General principles of wound healing. Surgical Clinics of North America 77 (3): 509.
Squarize, C.H., Castilho, R.M., Bugge, T.H., and Gutkind, J.S. (2010). Accelerated wound healing by mTOR activation in genetically defined mouse models. PloS One 5 (5): e10643.
Kluwe, J., A. Mencin, and R.F. Schwabe. 2009. Toll-like receptors, wound healing, and carcinogenesis. Journal of Molecular Medicine-JMM 87 (2): 125–138.
Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nature Reviews Immunology 1 (2): 135–145.
Kisseleva, T., et al. 2002. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 285 (1–2): 1–24.
Banno, T., A. Gazel, and M. Blumenberg. 2004. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. Journal of Biological Chemistry 279 (31): 32633–32642.
Xu, J., S. Lamouille, and R. Derynck. 2009. TGF-beta-induced epithelial to mesenchymal transition. Cell Research 19 (2): 156–172.
Cho, H.R., et al. 2004. Differential expression of TGF-beta isoforms during differentiation of HaCaT human keratinocyte cells: Implication for the separate role in epidermal differentiation. Journal of Korean Medical Science 19 (6): 853–858.
Tandara, A.A., and T.A. Mustoe. 2004. Oxygen in wound healing—More than a nutrient. World Journal of Surgery 28 (3): 294–300.
Shaikh, G., and B. Cronstein. 2016. Signaling pathways involving adenosine A(2A) and A(2B) receptors in wound healing and fibrosis. Purinergic Signalling 12 (2): 191–197.
Takao, J., et al. 2003. Expression of NF-kappa B in epidermis and the relationship between NF-kappa B activation and inhibition of keratinocyte growth. British Journal of Dermatology 148 (4): 680–688.
Morias, Y., et al. 2015. Ly6C-monocytes regulate parasite-induced liver inflammation by inducing the differentiation of pathogenic Ly6C+ monocytes into macrophages. Plos Pathogens 11 (5): e1004873.
Wajant, H., K. Pfizenmaier, and P. Scheurich. 2003. Tumor necrosis factor signaling. Cell Death and Differentiation 10 (1): 45–65.
Youssef, A., Aboalola, D. and Han, V.K., 2017. The roles of insulin-like growth factors in mesenchymal stem cell niche. Stem Cells International 2017: 12.
Huat, T.J., Khan, A.A., Pati, S., Mustafa, Z., Abdullah, J.M., and Jaafar, H. (2014). IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells. BMC Neuroscience 15 (1): 91.
Brons, I.G.M., et al. 2007. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448 (7150): 191–1U7.
Palmqvist, L., et al. 2005. Correlation of murine embryonic stem cell gene expression profiles with functional measures of pluripotency. Stem Cells 23 (5): 663–680.
Acknowledgements
We are grateful to Sobia Sadia for her assistance in language editing.
Funding
This work was supported by grants from Natural Science Foundation of China (No. 31371404, 31571429), Natural Science Foundation of Guangdong (2015A030311041), and Shenzhen Science and Technology Innovation Committee (GJHZ20150316160614842, JCY20160301150838144).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were performed with the approval of the Animal Ethics Committee of Tsinghua University.
Electronic Supplementary Material
Supplementary Table 1
(DOCX 37 kb)
Supplementary Table 2
(DOCX 49 kb)
Supplementary Table 3
(DOCX 47 kb)
Supplementary Figure 1
Gating and sorting of three types of inflammatory cells. Single cell suspensions derived from skin wound tissues were subjected to flow cytometry analysis. Plot was gated largely to avoid cell debrils (a). Residential macrophages were defined as F4/80+/CX3CR1+ cells, inflammatory macrophages were gated as F4/80+/Ly6C+ cells, and neutrophils were sorted for the F4/80-/Gr-1+ subset. Cells stained with corresponding isotype IgGs were used as a negative controls (b). (JPEG 10 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Wang, X., Chen, H. et al. Distinctively Expressed Cytokines by Three Different Inflammation Cells and Their Interaction with Keratinocytes in Wound Healing. Inflammation 40, 2151–2162 (2017). https://doi.org/10.1007/s10753-017-0655-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0655-9