Abstract
Toll-like receptors (TLRs) are innate pattern recognition receptors that play a critical role in allergic inflammation, yet their contribution to autophagy in asthma remains poorly defined. Here, we investigate the role of Toll-like receptor 2 (TLR2) in phosphoinositide 3-kinases/protein kinase B (PI3K/Akt) pathway-mediated autophagy in ovalbumin-induced airway inflammation in mice. Wild-type (WT) and TLR2-knockout (TLR2−/−) C57BL/6 mice were ovalbumin-sensitized and ovalbumin-challenged. In ovalbumin-challenged WT mice, enhanced expression of TLR2 in lung tissue, remarkable inflammatory cell infiltrates, goblet cell hyperplasia, and increased mucus production were observed. The number of inflammatory cells and interleukin-13 (IL-13) levels increased, while interferon-gamma (IFN-γ) levels decreased in bronchoalveolar lavage fluid. Expression of PI3K, phospho-Akt, Beclin-1 and LC3-II was enhanced significantly. These changes were mitigated dose-dependently in 3-methyl adenine-treated mice. In contrast, similar but weaker changes were found in ovalbumin-challenged TLR2−/− mice, and the changes were not significantly attenuated by 3-methyl adenine treatment. These results indicate that TLR2 confers a pivotal role in allergic airway inflammation via regulating the PI3K/Akt signaling pathway-related autophagy in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Asthma is an allergic inflammatory disorder of the airway. The allergic inflammation, which involves multiple inflammatory cells and mediators in the airways, persists even during symptom-free phase in asthma patients [1]. Both innate and adaptive immune abnormalities are considered to be implicated in asthma [2, 3].
Toll-like receptors (TLRs) are a group of primary sensors, which play an important role in both innate and adaptive immunity [ 4 ]. Increasing evidence has highlighted a central role of TLRs in the stimulation of innate and adaptive immunity and possibly in asthma [5, 6]. Toll-like receptor 2 (TLR2), one of the TLRs families, has been reported significantly over-expressed in asthma patients [7]. TLR2 could activate the innate immune system [8]. Activation of TLR2 could induce a Th2 immune response and promote experimental asthma [9]. TLR2 is also proven associated with the airway remodeling in asthma [10]. Depletion of TLR2 would alleviate the inflammation in experimental allergic mice [11]. Obviously, TLR2 might play an important role in the abnormal immune responses in asthma.
Autophagy is a cell death and survival pathway that differs from apoptosis [12]. In the past decade, emerging evidence has demonstrated that autophagy is directly relevant to many immunological and inflammatory diseases including a number of respiratory diseases such as embolism and pulmonary fibrosis [13]. Recently, autophagy was also found to be involved in asthma [14]. Meanwhile, how it is regulated in asthma remains largely unknown.
In this study, we investigated the role of TLR2 in the pathogenesis of OVA-induced allergic airway inflammation, concentrating on its effects on phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signaling pathway-related autophagy in a murine model.
METHODS
Animals
Female C57BL/6 wild-type (WT) mice were purchased from Shanghai Laboratory Animal Center (Shanghai, China). TLR2-knockout (TLR2−/−) mice on a C57BL/6 background were gifted by Dr. ZG. Tian (University of Science and Technology of China, Hefei, China). All mice were 6 to 7 weeks old in this study and housed in a standard specific pathogen-free conditioned animal care facility. A prior approval from the Animal Care and Use Committee of University of Science and Technology of China was obtained for this study.
Experiment Protocol and Treatment
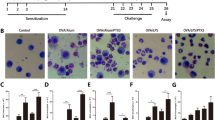
Mice were randomly assigned to the controls and experimental allergic inflammatory groups. The experimental groups were further divided into the OVA-treatment groups, OVA plus low dose of and high dose of 3-methyl adenine (3-MA) treatment groups. The mice in experimental groups were OVA-sensitized and OVA-challenged using a method previously reported [15], as illustrated in Fig. 1. An intraperitoneally injection with 10 μg OVA (Chicken Egg OVA, Grade V; Sigma) and 1 mg aluminum potassium sulfate in 0.5 ml saline were given on Day 0, and followed by 1% aerosolized OVA inhalation for 7 days from day 14 to 20; mice in OVA-3-MA treatment groups were injected intraperitoneally with 15 or 30 mg/kg of 3-MA 1.5 h before each OVA challenge. Twenty-four hours after the last challenge, mice were anesthetized with 3% pentobarbital sodium (50 mg/kg). Bronchoalveolar lavage (BAL) was performed as previously reported [16]. The BAL fluid was collected for cytological and cytokine examinations. Part of the right lung was used for histological examination, and the rest was frozen at −80 °C for western blotting analysis. Mice in the controls were treated similarly with intraperitoneal injection with aluminum potassium sulfate solution and followed by inhalation with saline instead of OVA solution.
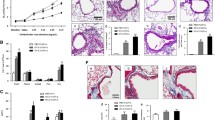
Study protocol, TLR2 expression, and inflammatory changes in the lung tissues after OVA challenge. a Study protocol. b, c Expression of TLR2 was increased in lung tissue of OVA-sensitized and OVA-challenged mice. d, e Histological score was lower in lung tissue of OVA-stimulated TLR2−/− mice than WT type of mice, H&E-staining, magnification ×200. f, g PAS+ cells were less in lung tissue of OVA-stimulated TLR2−/− mice, PAS-staining, magnification ×200. Representative lung tissue sections of at least three independent experiments are shown. *p < 0.05, **p < 0.01 versus WT control mice; #p < 0.05, ##p < 0.01 versus the TLR2−/− controls; $ p < 0.05 and $$ p < 0.01 indicates the differences between WT and TLR2−/− mice.
Histological Examination
Lung tissues were fixed in a 4% formaldehyde solution for 24 h and paraffin-embedded. Serial sections at 5 μm thickness were made and stained with hematoxylin and eosin (H&E) for airway inflammation observation or periodic acid-Schiff (PAS) for goblet cell hyperplasia and mucus production. The pathological changes were determined in a blinded fashion. An 8-point semi-quantitative scoring system for peribronchial inflammation in lung tissue was adopted [17]. Pierre Camateros’ method was referred to quantification of goblet cell hyperplasia [18]. Goblet cell hyperplasia within the bronchial epithelium was determined by counting PAS-positive cell numbers and standardized by dividing by the perimeter of the basement membrane.
Examination of BAL Fluid
The BAL fluid was centrifuged at 1000 rpm in 4 °C, and the cell pellet was re-suspended with phosphate buffered solution. Total cell counts were determined using a hemocytometer. Wright-staining was used for cell differential counting which was performed in a blinded manner. The levels of interleukin-13 (IL-13) and interferon-gamma (IFN-γ) were separately measured using commercially available ELISA kits (Cusabio, Wuhan, China) in accordance with the manufacturer’s instruction manual.
Western Blot Analysis
Lung tissues were homogenized and total protein concentrations were determined using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Proteins were separated on 12% SDS-PAGE gels and transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked in 5% non-fat milk for 1 h at room temperature and probed with antibodies against TLR2 (Millipore, Billerica, MA, USA), MyD88 (Abcam, Boston, MA, USA), PI3K (Sigma-Aldrich, St. Louis, MO, USA), Akt and Ser-473 phosphorylated Akt (p-Akt) (Cell Signaling Technology, Boston, MA, USA), Beclin-1 (Abcam, Boston, MA, USA), and microtubule-associated protein light chain 3 (LC3) (Novus Biologicals, Littleton, CO, USA). GAPDH (Kangchen Biotech, Beijing, China) was used as the loading control. The intensity of protein bands was digitized by using an Image J 1.38× software (NIH, Bethesda, MD, USA).
Statistical Analysis
Data were presented as mean ± standard error of mean (SEM). Experiments were repeated at least three times. Prism software (GraphPad Software5.0, Inc., San Diego, CA) was used for statistical calculations. An independent sample t test or one-way ANOVA followed by a Bonferroni post-test procedure were used. Statistical significance was set at p < 0.05.
RESULTS
TLR2 Expression and Inflammatory Changes in Lung Tissue
We first detected whether TLR2 expression changes in mice received OVA sensitization and challenge. Western blot results revealed that expression of TLR2 in the lung tissue lysates in OVA-stimulated mice was significantly increased (Fig. 1b, c). Second, we compared the histological changes in lung tissues of WT and TLR2−/− mice after OVA challenge, found significant differences between these two types of mice. In the lung tissues of OVA-challenged WT mice, significant inflammatory cell infiltrates (Fig. 1d, e), mucus plugs in the cavity of bronchioles (Fig. 1f), and increased PAS-positive cells (Fig. 1g) were detected. Contrarily, in OVA-challenged TLR2−/− mice, these inflammatory changes were relatively weaker (Fig. 1).
Cytological and Cytokine Changes in BAL Fluid
A marked increase of total cell numbers was found in OVA-sensitized and OVA-challenged WT mice. Inflammatory cells including eosinophils, neutrophils, and monocytes in the BAL fluid were substantially increased. By contrast, in OVA-challenged TLR2−/− mice, the total number of inflammatory cells was also significantly increased compared with that of control TLR2−/− mice, but not as high as that in OVA-stimulated WT mice. A statistically significant difference existed between the WT and TLR2−/− mice. Similar differences were found in the changes of cytokines between the two groups. From OVA-challenged WT mice, IL-13 levels in BAL fluids increased and IFN-γ levels decreased significantly relative to the controls. While In OVA-challenged TLR2−/− mice, similar but weaker changes were presented (Fig. 2).
Cell number and cytokine changes in BAL fluid. Data are presented as mean ± SEM (n = 7 each group). *p < 0.05, **p < 0.01 versus the control WT mice; #p < 0.05, ##p < 0.01 versus the TLR2−/− controls; $p < 0.05 and $$p < 0.01 indicates the differences between OVA-sensitized and OVA-challenged WT and TLR2−/− mice.
Expression of PI3K/P-Akt Signaling Pathway Proteins in Lung Tissue Lysates
Western blot analysis showed that expression of MyD88 was similarly elevated in both WT and TLR2−/− mice after OVA sensitization and challenge. The expression of PI3K, P-Akt, Beclin-1, and LC3-II was increased significantly in OVA-challenged WT mice. By contrast, in OVA-stimulated TLR2−/− mice, the expression of proteins increased remarkably compared with that of the control TLR2−/− mice, but not as strong as that in the WT ones. The differences induced by OVA challenge between WT and TLR2−/− mice were statistically significant (Fig. 3).
Protein expression in lung tissue lysates. The differences of protein expression in lung tissue lysates between wild and TLR2−/− mice after OVA sensitization and challenge are shown. *p < 0.05, **p < 0.01 versus the WT controls; #p < 0.05, ##p < 0.01 versus the TLR2−/− controls; $p < 0.05 and $$p < 0.01 indicates the differences between OVA-sensitized and OVA-challenged WT and TLR2−/− mice.
Inflammatory Changes in the Lung Tissues of Mice Received 3-MA Treatment
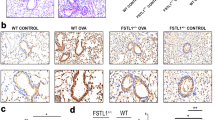
Inflammatory changes in lung tissues were significantly attenuated in 3-MA-treated OVA-stimulated WT mice. H&E-stained lung tissue slices presented a statistically significant alleviation of inflammation in WT mice processed with high doses of 3-MA. The mucus hypersecretion and PAS-positive cell numbers were considerably reduced by low doses of 3-MA treatment. The reduction was more remarkable in mice received higher doses of 3-MA. Contrarily, no statistically significant attenuation of inflammation and goblet cell hyperplasia in 3-MA-treated TLR2−/− mice were observed (Fig. 4).
Inflammatory changes in mice treated with 3-MA. a, b showed the histological score changes in low or high doses of 3-MA-treated wild or TLR2−/− mice, HE-stain, magnification ×200; c, d changes of number of PAS+ cells in low or high doses of 3-MA-treated mice, PAS-stain, magnification: 200×; **p < 0.01 versus the OVA-challenged mice.
Cytological and Cytokine Changes in BAL Fluid in 3-MA-Treated Mice
The total number of inflammatory cells in BAL fluid increased substantially in the experimental allergic WT mice compared with that of the controls. The total number of cells especially the eosinophils was decreased in mice treated with low doses of 3-MA. High doses of 3-MA treatment conferred a further reduction of the neutrophils and monocytes. Compared with these changes, the reduction of inflammatory cells was not statistically significant in 3-MA-treated TLR2−/− mice. Besides, the cytokines in the BAL fluid changed concomitantly. In 3-MA-treated experimental allergic WT mice, the increased level of IL-13 was decreased, and INF-γ level elevated relative to the controls. The changes were not significant in mice accepted low doses of 3-MA treatment but statistically significant in mice received high doses of 3-MA administration. Comparatively, the changes were not statistically significant in TLR2−/− mice administered with neither low nor high doses of 3-MA (Fig. 5).
Protein Expression in Lung Tissue Lysates in 3-MA-Treated Mice
Western blotting and semi-quantitative analysis showed that the expression of PI3K, p-Akt, Beclin-1, and LC3-II was significantly enhanced in the lung tissue of OVA-sensitized and OVA-challenged WT mice. The increased expression was downregulated in mice that received low doses of 3-MA treatment; higher doses of 3-MA administration resulted in a more remarkable downregulation. These results demonstrated a dose-dependent inhibitory effect of 3-MA on the expression of PI3K/Akt pathway proteins (Fig. 6). Contrarily, in OVA-challenged TLR2−/− mice, expression of PI3K, p-Akt, Beclin-1, and LC3-II was not significant changed under neither low nor high doses of 3-MA treatment, which was significantly different from the that in WT ones (p < 0.05, Fig. 7).
DISCUSSION
TLR2 is one of the TLRs which can recognize a variety of ligands from pathogens [19] to endogenous alarmins [20] in initiating immunological responses. It was reported that TLR2 was involved in airway allergic diseases [21, 22], as markedly upregulated in the airway tissues following allergen challenge [23]. Researches indicated that TLR2 was associated with the prevalence of post-bronchiolitis asthma [24] and the susceptibility to asthma in children [25]. However, the role of TLR2 in the pathogenesis of asthma has been debated. Some studies demonstrated that activation of TLR2 could reduce the allergic airway inflammation [26] and protect against allergic asthma upon subsequent allergen provocation [16]. Meanwhile, others concluded that TLR2 contributed to the acute inflammation in asthma [27], acted as a major contributor to the inflammatory in asthma [11]. In our study, a remarkable upregulated TLR2 expression in the lung tissues of OVA-stimulated WT mice were observed (Fig. 1b, c), accompanied with marked inflammatory changes in lung tissue and significant increase of inflammatory cells in BAL fluid (Fig. 2). By comparison, in TLR2−/− mice, the inflammatory responses, inflammatory cell infiltrates, and cytokine changes were weaker under OVA challenge.
It is well-known that the development of helper T cell immunity is shaped by activation of pattern recognition receptors, and a Th1/Th2 imbalance is existed in asthma [11, 28, 29]. TLR2 mediates the innate immune response of alveolar macrophage to allergen [30]. In mice model of OVA-induced asthma, TLR2 is a major contributor to the maintenance of adaptive Th2-cytokine-driven inflammation [11]. In our study, a more remarkable increase of the levels of IL-13, a Th2 cytokine, and decrease of IFN-γ were found in OVA-stimulated WT mice, compared with the weaker cytokine changes in TLR2−/− mice, supported the Th1/Th2 imbalance hypothesis, and implied the necessity of TLR2 for proper immune inflammatory response in allergen-induced inflammation. These cytokine abnormalities, together with the inflammatory changes, supported the conclusions of the others that TLR2 plays a pivotal role in allergic inflammation [31] and contributes to asthma [11].
Autophagy is an evolutionarily conserved physical process with significant impact on immunity response specifically Th2 immune response in allergic inflammation [32]. Although it has been extensively studied in many other diseases, autophagy was yet merely suggested being implicated in asthma by a few recent studies [14, 32]. PI3K is a key kinase in the autophagy signaling pathways which can trigger the serine phosphorylation of Akt and promote its downstream substrates Beclin-1 and LC-3 to form the autophagosome [33]. In our study, a marked upregulation of expression of PI3K, accompanied with increased expression of p-Akt, Beclin-1, and LC3-II in OVA-challenged WT mice was observed. By contrast, in TLR2−/− mice, expression of these proteins was elevated but significantly weaker than that in the WT ones. These results suggested the TLR2 might be necessary for the proper function of PI3K/Akt pathway-mediated autophagy.
Researches demonstrated that PI3Ks can regulate the activation of multiple intracellular signaling cascades and function of many inflammatory mediators [34, 35]. In asthma, PI3Ks regulate many of the inflammatory cytokines [36] and the recruitment and activation of neutrophils, eosinophils, and macrophage [37]. In our study, in OVA-challenged WT mice, concomitant with the enhanced expression of PI3K-pathway proteins, an enhanced inflammatory cell infiltration in lung tissue and IL-13/IFN-γ cytokine imbalance, were observed. Contrarily, in TLR2−/− mice, weaker airway inflammation, cytokine imbalance, and protein expression were found. These results implied that the PI3K/Akt pathway was involved in the allergic inflammation, and this autophagous pathway might be TLR2 dependent. In fact, a TLR2/PI3k signaling pathway has been suggested by several recent studies. Li et al. reported that inhibition of TLR2 almost blocked the PI3K/Akt signaling pathway in protein synthesis [38]. In mouse macrophages, the activation of PI3K/Akt pathway was TLR2-dependent [39]. Activation of TLR2/PI3K/Akt signaling pathway was even found crucial important in mycobacteria [40]. Our results were consistent with the conclusions of these studies.
To further explore whether TLR2 was crucial to PI3K/Akt signaling pathway-mediated autophagy, we treated the mice with low or high doses of 3-MA, a commonly used reagent which could inhibit PI3K, block the autophagosome formation, and consequently inhibit the autophagy [41]. The expression of PI3K, p-Akt, Beclin-1, and LC3-II was downregulated in OVA-challenged WT mice (Fig. 6), accompanied with an alleviation of inflammatory cell infiltrates and IL-13/IFN-γ imbalance (Figs. 5, 6), which agreed with the results of other studies [42, 43]. In comparison, in TLR2−/− mice, neither low nor high doses of 3-MA treatment resulted in significant changes as observed in WT ones. Apparently, 3-MA treatment did not exert remarkable inhibitory effects when TLR2 was absent. These results manifested that TLR2 was needed for the proper functioning of PI3K/Akt signaling pathway in autophagy in OVA-induced allergic inflammatory mice.
It is noted that airway inflammation and PI3k/Akt pathway-mediated autophagy were weaker but not absent in TLR2−/− mice. Besides, the expression of adaptor protein MyD88 enhanced similarly in both WT and TLR2−/− mice after OVA challenge. These might be due to the complexity of signal pathways involved. In fact, except for TLR2, other TLRs such as TLR4, 7, and 9 are also involved in asthma [44]. For example, both TLR4 [45, 46] and TLR7 [47] contribute to asthma MyD88 dependently. These TLRs can co-regulate many of the physiological processes including the inflammatory process and autophagy [6, 48]. Obviously, further studies on the role of TLRs in the autophagy in asthma are needed.
Taken together, our study revealed that TLR2 played a pivotal role in OVA-induced inflammation in mice, partly by regulating the PI3K/Akt signaling pathway-related autophagy. Blockage of TLR2/PI3K/Akt signaling pathway could attenuate the inflammation, which suggested a potentially therapeutic target for the treatment of asthma.
References
Anderson, G.P. 2008. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 372: 1107–1119.
Holtzman, M.J. 2012. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. The Journal of Clinical Investigation 122: 2741–2748.
Holgate, S.T. 2012. Innate and adaptive immune responses in asthma. Nature Medicine 18: 673–683.
Goulopoulou, S., C.G. McCarthy, and R.C. Webb. 2016. Toll-like receptors in the vascular system: sensing the dangers within. Pharmacological Reviews 68: 142–167.
Larsen, J.M., H.S. Musavian, T.M. Butt, C. Ingvorsen, A.H. Thysen, and S. Brix. 2015. Chronic obstructive pulmonary disease and asthma-associated Proteobacteria, but not commensal Prevotella spp., promote Toll-like receptor 2-independent lung inflammation and pathology. Immunology 144: 333–342.
Shimizu, T., Y. Kimura, Y. Kida, K. Kuwano, M. Tachibana, M. Hashino, et al. 2014. Cytadherence of Mycoplasma pneumoniae induces inflammatory responses through autophagy and toll-like receptor 4. Infection and Immunity 82: 3076–3086.
Sanchez-Zauco, N., B. Del Rio-Navarro, C. Gallardo-Casas, J. Del Rio-Chivardi, R. Muriel-Vizcaino, C. Rivera-Pazos, et al. 2014. High expression of Toll-like receptors 2 and 9 and Th1/Th2 cytokines profile in obese asthmatic children. Allergy and Asthma Proceedings 35: 34–41.
Page, K., K.M. Lierl, V.S. Hughes, P. Zhou, J.R. Ledford, and M. Wills-Karp. 2008. TLR2-mediated activation of neutrophils in response to German cockroach frass. Journal of Immunology 180: 6317–6324.
Redecke, V., H. Hacker, S.K. Datta, A. Fermin, P.M. Pitha, D.H. Broide, et al. 2004. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. Journal of Immunology 172: 2739–2743.
Page, K., J.R. Ledford, P. Zhou, and M. Wills-Karp. 2009. A TLR2 agonist in German cockroach frass activates MMP-9 release and is protective against allergic inflammation in mice. Journal of Immunology 183: 3400–3408.
Li, X., Q. Chen, C. Chu, H. You, M. Jin, X. Zhao, et al. 2014. Ovalbumin-induced experimental allergic asthma is Toll-like receptor 2 dependent. Allergy and Asthma Proceedings 35: e15–e20.
Chaabane, W., S.D. User, M. El-Gazzah, R. Jaksik, E. Sajjadi, J. Rzeszowska-Wolny, et al. 2013. Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Archivum Immunologiae et Therapiae Experimentalis (Warsz) 61: 43–58.
Mizumura, K., S.M. Cloonan, J.A. Haspel, and A.M. Choi. 2012. The emerging importance of autophagy in pulmonary diseases. Chest 142: 1289–1299.
Poon, A.H., F. Chouiali, S.M. Tse, A.A. Litonjua, S.N. Hussain, C.J. Baglole, et al. 2012. Genetic and histologic evidence for autophagy in asthma pathogenesis. The Journal of Allergy and Clinical Immunology 129: 569–571.
Locke, N.R., S.G. Royce, J.S. Wainewright, C.S. Samuel, and M.L. Tang. 2007. Comparison of airway remodeling in acute, subacute, and chronic models of allergic airways disease. American Journal of Respiratory Cell and Molecular Biology 36: 625–632.
Nawijn, M.C., A.C. Motta, R. Gras, S. Shirinbak, H. Maazi, and A.J. van Oosterhout. 2013. TLR-2 activation induces regulatory T cells and long-term suppression of asthma manifestations in mice. PloS One 8: e55307.
Koltsida, O., S. Karamnov, K. Pyrillou, T. Vickery, A.D. Chairakaki, C. Tamvakopoulos, et al. 2013. Toll-like receptor 7 stimulates production of specialized pro-resolving lipid mediators and promotes resolution of airway inflammation. EMBO Molecular Medicine 5: 762–775.
Camateros, P., M. Tamaoka, M. Hassan, R. Marino, J. Moisan, D. Marion, et al. 2007. Chronic asthma-induced airway remodeling is prevented by toll-like receptor-7/8 ligand S28463. American Journal of Respiratory and Critical Care Medicine 175: 1241–1249.
de Aquino, S.G., S. Abdollahi-Roodsaz, M.I. Koenders, F.A. van de Loo, G.J. Pruijn, R.J. Marijnissen, et al. 2014. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. Journal of Immunology 192: 4103–4111.
Thierry, A., S. Giraud, A. Robin, A. Barra, F. Bridoux, V. Ameteau, et al. 2014. The alarmin concept applied to human renal transplantation: evidence for a differential implication of HMGB1 and IL-33. PloS One 9: e88742.
He M, Ichinose T, Song Y, Yoshida Y, Bekki K, Arashidani K, et al. 2016. Desert dust induces TLR signaling to trigger Th2-dominant lung allergic inflammation via a MyD88-dependent signaling pathway. Toxicol Appl Pharmacol.
Pace, E., C. Di Sano, M. Ferraro, A. Bruno, V. Caputo, S. Gallina, et al. 2015. Budesonide increases TLR4 and TLR2 expression in Treg lymphocytes of allergic asthmatics. Pulmonary Pharmacology & Therapeutics 32: 93–100.
Fransson, M., M. Adner, J. Erjefalt, L. Jansson, R. Uddman, and L.O. Cardell. 2005. Up-regulation of Toll-like receptors 2, 3 and 4 in allergic rhinitis. Respiratory Research 6: 100.
Koponen, P., J. Vuononvirta, K. Nuolivirta, M. Helminen, Q. He, and M. Korppi. 2014. The association of genetic variants in toll-like receptor 2 subfamily with allergy and asthma after hospitalization for bronchiolitis in infancy. The Pediatric Infectious Disease Journal 33: 463–466.
Kerkhof, M., D.S. Postma, B. Brunekreef, N.E. Reijmerink, A.H. Wijga, J.C. de Jongste, et al. 2010. Toll-like receptor 2 and 4 genes influence susceptibility to adverse effects of traffic-related air pollution on childhood asthma. Thorax 65: 690–697.
Fuchs, B., S. Knothe, S. Rochlitzer, M. Nassimi, M. Greweling, H.D. Lauenstein, et al. 2010. A Toll-like receptor 2/6 agonist reduces allergic airway inflammation in chronic respiratory sensitisation to Timothy grass pollen antigens. International Archives of Allergy and Immunology 152: 131–139.
Ferreira, D.S., R. Annoni, L.F. Silva, M. Buttignol, A.B. Santos, M.C. Medeiros, et al. 2012. Toll-like receptors 2, 3 and 4 and thymic stromal lymphopoietin expression in fatal asthma. Clinical and Experimental Allergy 42: 1459–1471.
Shieh, Y.H., H.M. Huang, C.C. Wang, C.C. Lee, C.K. Fan, and Y.L. Lee. 2015. Zerumbone enhances the Th1 response and ameliorates ovalbumin-induced Th2 responses and airway inflammation in mice. International Immunopharmacology 24: 383–391.
Barboza, R., N.O. Camara, E. Gomes, A. Sa-Nunes, E. Florsheim, L. Mirotti, et al. 2013. Endotoxin exposure during sensitization to allergens shifts TH2 immunity towards a TH17-mediated airway neutrophilic inflammation: role of TLR4 and TLR2. PloS One 8: e67115.
Liu, C.F., D. Drocourt, G. Puzo, J.Y. Wang, and M. Riviere. 2013. Innate immune response of alveolar macrophage to house dust mite allergen is mediated through TLR2/−4 co-activation. PloS One 8: e75983.
Buckland, K.F., E. O'Connor, L.A. Murray, and C.M. Hogaboam. 2008. Toll like receptor-2 modulates both innate and adaptive immune responses during chronic fungal asthma in mice. Inflammation Research 57: 379–387.
Jyothula, S.S., and N.T. Eissa. 2013. Autophagy and role in asthma. Current Opinion in Pulmonary Medicine 19: 30–35.
He, C., and B. Levine. 2010. The beclin 1 interactome. Current Opinion in Cell Biology 22: 140–149.
Xuan F, Jian J, Lin X, Huang J, Jiao Y, Huang W, et al. 2016. 17-Methoxyl-7-Hydroxy-Benzene-Furanchalcone Ameliorates Myocardial Ischemia/Reperfusion Injury in Rat by Inhibiting Apoptosis and Autophagy Via the PI3K-Akt Signal Pathway. Cardiovasc Toxicol.
Yang, J., Q. Chen, S. Tian, S. Song, F. Liu, Q. Wang, et al. 2015. The role of 1,25-dyhydroxyvitamin D3 in mouse liver ischemia reperfusion injury: regulation of autophagy through activation of MEK/ERK signaling and PTEN/PI3K/Akt/mTORC1 signaling. American Journal of Translational Research 7: 2630–2645.
Nashed, B.F., T. Zhang, M. Al-Alwan, G. Srinivasan, A.J. Halayko, K. Okkenhaug, et al. 2007. Role of the phosphoinositide 3-kinase p110delta in generation of type 2 cytokine responses and allergic airway inflammation. European Journal of Immunology 37: 416–424.
Condliffe, A.M., K.A. Cadwallader, T.R. Walker, R.C. Rintoul, A.S. Cowburn, and E.R. Chilvers. 2000. Phosphoinositide 3-kinase: a critical signalling event in pulmonary cells. Respiratory Research 1: 24–29.
Jing Y, Cai X, Xu Y, Zhu C, Wang L, Wang S, et al. 2016. alpha-Lipoic acids promote the protein synthesis of C2C12 myotubes by the TLR2/PI3K signaling pathway. J Agric Food Chem.
Liu Y, Li JY, Chen ST, Huang HR, Cai H. 2015. The rLrp of mycobacterium tuberculosis inhibits proinflammatory cytokine production and downregulates APC function in mouse macrophages via a TLR2-mediated PI3K/Akt pathway activation-dependent mechanism. Cell Mol Immunol.
Liu, H., Z. Liu, J. Chen, L. Chen, X. He, R. Zheng, et al. 2013. Induction of CCL8/MCP-2 by mycobacteria through the activation of TLR2/PI3K/Akt signaling pathway. PloS One 8: e56815.
AKT. 2013. Serine/threonine protein kinase modulates baicalin-triggered autophagy in human bladder cancer T24 cells. International Journal of Oncology 42: 993–1000.
Cheng, C., W.E. Ho, F.Y. Goh, S.P. Guan, L.R. Kong, W.Q. Lai, et al. 2011. Anti-malarial drug artesunate attenuates experimental allergic asthma via inhibition of the phosphoinositide 3-kinase/Akt pathway. PloS One 6: e20932.
Lee, K.S., H.K. Lee, J.S. Hayflick, Y.C. Lee, and K.D. Puri. 2006. Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. The FASEB Journal 20: 455–465.
Sykes, A., M.R. Edwards, J. Macintyre, A. Del Rosario, V. Gielen, J. Haas, et al. 2013. TLR3, TLR4 and TLRs7-9 induced interferons are not impaired in airway and blood cells in well controlled asthma. PloS One 8: e65921.
Arora, M., S.L. Poe, T.B. Oriss, N. Krishnamoorthy, M. Yarlagadda, S.E. Wenzel, et al. 2010. TLR4/MyD88-induced CD11b+Gr-1 in. F4/80+ non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunology 3: 578–593.
Yang, M., R.K. Kumar, and P.S. Foster. 2009. Pathogenesis of steroid-resistant airway hyperresponsiveness: interaction between IFN-gamma and TLR4/MyD88 pathways. Journal of Immunology 182: 5107–5115.
Moisan, J., P. Camateros, T. Thuraisingam, D. Marion, H. Koohsari, P. Martin, et al. 2006. TLR7 ligand prevents allergen-induced airway hyperresponsiveness and eosinophilia in allergic asthma by a MYD88-dependent and MK2-independent pathway. American Journal of Physiology. Lung Cellular and Molecular Physiology 290: L987–L995.
Oka, T., S. Hikoso, O. Yamaguchi, M. Taneike, T. Takeda, T. Tamai, et al. 2012. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485: 251–255.
Acknowledgments
We are grateful to Dr. Jiang-Ning Zhou from the University of Science and Technology of China for the technical assistance. We would like to thank Dr. Zhi-Gang Tian from the University of Science and Technology of China for TLR2−/− mice. This study was supported by the Natural Science Foundation of China (No. 81170030, 81270082, and 81300027), National Education Ministry of China (No. 20113420110006), Annual Research Project of Anhui Province (No. 10021303028), and Key Lab of Geriatric Molecular Medicine of Anhui Province (No. 1206c0805028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jiang, X., Fang, L., Wu, H. et al. TLR2 Regulates Allergic Airway Inflammation and Autophagy Through PI3K/Akt Signaling Pathway. Inflammation 40, 1382–1392 (2017). https://doi.org/10.1007/s10753-017-0581-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0581-x