Abstract
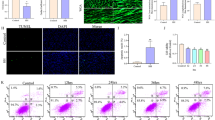
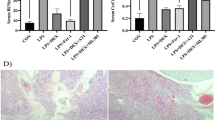
Acute lung injury caused by cardiopulmonary bypass (CPB) increases the mortality after cardiac surgery. Our previous clinical study suggested that electroacupuncture (EAc) has a protective effect during CPB, but the mechanism was unclear. So, we design this study to investigate the effects of EAc on CPB-induced lung injury and the underlying mechanism. Male Sprague Dawley rats were randomly divided into control, CPB, and CPB + EAc groups. A lung injury model was created by CPB surgery to serve as the CPB group, and EAc (2/100 Hz) was used before CPB in the CPB + EAc group. Lung tissue was collected at 0.5, 1, and 2 h after CPB. Pulmonary malondialdehyde (MDA) concentrations as well as superoxide dismutase (SOD), myeloperoxidase (MPO), and caspase-3 activity were determined. c-Jun N-terminal kinase (JNK), ERK, p38 and cleaved caspase 3 in the lung were analyzed by western blotting. A549 cells were treated by rat serum from the CPB and CPB + EAc groups, and cleaved caspase-3 activity was detected by fluorescent immunohistochemistry. CPB significantly increased the MPO activity, MDA content, apoptosis, caspase-3 activity, and phosphorylated p38 but decreased SOD activity compared with the control group. EAc significantly increased SOD activity at 0.5 and 2 h (p < 0.01 vs CPB) and reduced CPB-induced histological changes, MPO activity at 1 and 2 h (p < 0.05 vs CPB), MDA content at 2 h (p < 0.05 vs CPB), caspase-3 activity at 1 h (p < 0.05 vs CPB), and phosphorylated p38 and JNK at 0.5 h after CPB. The serum from the CPB group increased more positive staining cells of cleaved caspase-3 than that from the CPB + EAc group. EAc reversed the CPB-induced lung inflammation, oxidative damage, and apoptosis; the mechanism may involve decreased phosphorylation of p38 along with caspase-3 activity and activation.





Similar content being viewed by others
References
Zakkar, M., G. Guida, M.S. Suleiman, and G.D. Angelini. 2015. Cardiopulmonary bypass and oxidative stress. Oxidative Medicine and Cellular Longevity 2015: 189863.
Pelosi, A., L.K. Anderson, J. Paugh, S. Robinson, and G.E. Eyster. 2013. Challenges of cardiopulmonary bypass–a review of the veterinary literature. Veterinary Surgery 42: 119–136.
Paparella, D., T.M. Yau, and E. Young. 2002. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. European Journal of Cardio-Thoracic Surgery 21: 232–244.
Murkin, J.M. 1997. Cardiopulmonary bypass and the inflammatory response: a role for serine protease inhibitors? Journal of Cardiothoracic and Vascular Anesthesia 11: 19–23 discussion 24-5.
Huffmyer, J.L., and D.S. Groves. 2015. Pulmonary complications of cardiopulmonary bypass. Best Practice & Research. Clinical Anaesthesiology 29: 163–175.
Cheng, S.B., and L.K. Ding. 1973. Practical application of acupuncture analgesia. Nature 242: 559–560.
Ernst, E. 2009. Acupuncture: what does the most reliable evidence tell us? Journal of Pain and Symptom Management 37: 709–714.
Cressey, D. 2010. Acupuncture for mice. Nature 465: 538.
Burnstock, G. 2009. Acupuncture: a novel hypothesis for the involvement of purinergic signalling. Medical Hypotheses 73: 470–472.
Goldman, N., M. Chen, T. Fujita, Q. Xu, W. Peng, W. Liu, et al. 2010. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nature Neuroscience 13: 883–888.
Kim, H.Y., J. Wang, I. Lee, H.K. Kim, K. Chung, and J.M. Chung. 2009. Electroacupuncture suppresses capsaicin-induced secondary hyperalgesia through an endogenous spinal opioid mechanism. Pain 145: 332–340.
Zhou, J., H. Chi, T.O. Cheng, T.Y. Chen, Y.Y. Wu, W.X. Zhou, et al. 2011. Acupuncture anesthesia for open heart surgery in contemporary China. International Journal of Cardiology 150: 12–16.
Engels, M., E. Bilgic, A. Pinto, E. Vasquez, L. Wollschlager, H. Steinbrenner, et al. 2014. A cardiopulmonary bypass with deep hypothermic circulatory arrest rat model for the investigation of the systemic inflammation response and induced organ damage. J Inflamm (Lond) 11: 26.
Li, W., B. Zheng, H. Xu, Y. Deng, S. Wang, X. Wang, et al. 2013. Isoflurane prevents neurocognitive dysfunction after cardiopulmonary bypass in rats. Journal of Cardiothoracic and Vascular Anesthesia 27: 502–509.
Matute-Bello, G., G. Downey, B.B. Moore, S.D. Groshong, M.A. Matthay, A.S. Slutsky, et al. 2011. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. American Journal of Respiratory Cell and Molecular Biology 44: 725–738.
Bradley, P.P., D.A. Priebat, R.D. Christensen, and G. Rothstein. 1982. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. The Journal of Investigative Dermatology 78: 206–209.
Chen, Y., J. Liu, S. Wang, B. Ji, Y. Tang, A. Wu, et al. 2012. Early changes in cerebral oxidative stress and apoptotic neuronal injury after various flows for selective cerebral perfusion in piglets. Perfusion 27: 419–425.
Yang, B., D. Ye, and Y. Wang. 2013. Caspase-3 as a therapeutic target for heart failure. Expert Opinion on Therapeutic Targets 17: 255–263.
Mazumder, S., D. Plesca, and A. Almasan. 2008. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods in Molecular Biology 414: 13–21.
Ni, X., Y. Xie, Q. Wang, H. Zhong, M. Chen, F. Wang, et al. 2012. Cardioprotective effect of transcutaneous electric acupoint stimulation in the pediatric cardiac patients: a randomized controlled clinical trial. Paediatric Anaesthesia 22: 805–811.
Fu, S.P., S.Y. He, B. Xu, C.J. Hu, S.F. Lu, W.X. Shen, et al. 2014. Acupuncture promotes angiogenesis after myocardial ischemia through H3K9 acetylation regulation at VEGF gene. PloS One 9: e94604.
Gao, J., W. Fu, Z. Jin, and X. Yu. 2007. Acupuncture pretreatment protects heart from injury in rats with myocardial ischemia and reperfusion via inhibition of the beta(1)-adrenoceptor signaling pathway. Life Sciences 80: 1484–1489.
Royston, D., B.D. Minty, T.W. Higenbottam, J. Wallwork, and G.J. Jones. 1985. The effect of surgery with cardiopulmonary bypass on alveolar-capillary barrier function in human beings. The Annals of Thoracic Surgery 40: 139–143.
Asimakopoulos, G., P.L. Smith, C.P. Ratnatunga, and K.M. Taylor. 1999. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. The Annals of Thoracic Surgery 68: 1107–1115.
Thompson, C.B. 1995. Apoptosis in the pathogenesis and treatment of disease. Science 267: 1456–1462.
Owais, K., T. Huang, F. Mahmood, J. Hubbard, R. Saraf, A. Bardia, et al. 2015. Cardiopulmonary bypass decreases activation of the signal transducer and activator of transcription 3 (STAT3) pathway in diabetic human myocardium. The Annals of Thoracic Surgery 100: 1636–1645.
Stassano, P., L. Di Tommaso, M. Monaco, G. Mastrogiovanni, A. Musumeci, A. Contaldo, et al. 2010. Left heart pump-assisted myocardial revascularization favorably affects neutrophil apoptosis. World Journal of Surgery 34: 652–657.
Yeh, C.H., T.P. Chen, Y.C. Wang, Y.M. Lin, and P.J. Lin. 2009. HO-1 activation can attenuate cardiomyocytic apoptosis via inhibition of NF-kappaB and AP-1 translocation following cardiac global ischemia and reperfusion. The Journal of Surgical Research 155: 147–156.
Yeh, C.H., T.P. Chen, C.H. Lee, Y.C. Wu, Y.M. Lin, and Lin P. Jing. 2006. Inhibition of poly(adp-ribose) polymerase reduces cardiomyocytic apoptosis after global cardiac arrest under cardiopulmonary bypass. Shock 25: 168–175.
Goebel, U., M. Siepe, A. Mecklenburg, P. Stein, M. Roesslein, C.I. Schwer, et al. 2008. Carbon monoxide inhalation reduces pulmonary inflammatory response during cardiopulmonary bypass in pigs. Anesthesiology 108: 1025–1036.
Qu, X., Q. Li, X. Wang, X. Yang, and D. Wang. 2013. N-acetylcysteine attenuates cardiopulmonary bypass-induced lung injury in dogs. Journal of Cardiothoracic Surgery 8: 107.
Acioli, P.C., O. Albuquerque Ade, I.B. Guimaraes, R.W. Araujo, P.R. Vasconcelos, and S.B. Guimaraes. 2014. Protective effects of abdominal electroacupuncture on oxidative stress and inflammation due to testis torsion/detorsion in rats. Acta Cirúrgica Brasileira 29: 450–456.
Xia, Z., M. Dickens, J. Raingeaud, R.J. Davis, and M.E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331.
Liu, S., X.P. Zhang, N.N. Han, S. Lv, and J.Y. Xiong. 2015. Pretreatment with low dose etomidate prevents etomidate-induced rat adrenal insufficiency by regulating oxidative stress-related MAPKs and apoptosis. Environmental Toxicology and Pharmacology 39: 1212–1220.
Zhong, L.R., X. Chen, and K.M. Wei. 2013. Radix tetrastigma hemsleyani flavone induces apoptosis in human lung carcinoma a549 cells by modulating the MAPK pathway. Asian Pacific Journal of Cancer Prevention 14: 5983–5987.
Schuh, K., and A. Pahl. 2009. Inhibition of the MAP kinase ERK protects from lipopolysaccharide-induced lung injury. Biochemical Pharmacology 77: 1827–1834.
Uhlig, U., J.J. Haitsma, T. Goldmann, D.L. Poelma, B. Lachmann, and S. Uhlig. 2002. Ventilation-induced activation of the mitogen-activated protein kinase pathway. The European Respiratory Journal 20: 946–956.
Ding, J., Q. Zhang, Q. Luo, Y. Ying, Y. Liu, Y. Li, et al. 2016. Alda-1 attenuates lung ischemia-reperfusion injury by reducing 4-hydroxy-2-nonenal in alveolar epithelial cells. Critical Care Medicine 44: e544–e552.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81202748 to CHS, 81170536 to LZG, and 81570056 to THF).
Author information
Authors and Affiliations
Contributions
CHS, THF, MW, and LZG designed the study and wrote the manuscript. MW, LZ, YZW, and ZJ prepared the CPB model and harvested the lung samples. LZG and TWL completed the determination of WB, MPO, and MDA. MW, LZG, and ZZW did the histology, and LZG and LYJ determined the activation of caspase3 in lung and cell. All authors approved the final version of the paper.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ma, W., Li, Z., Lu, Z. et al. Protective Effects of Acupuncture in Cardiopulmonary Bypass-Induced Lung Injury in Rats. Inflammation 40, 1275–1284 (2017). https://doi.org/10.1007/s10753-017-0570-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0570-0