Abstract
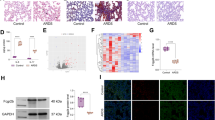
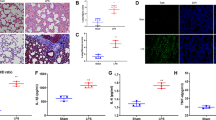
In the lungs, endothelial nitric oxide synthase (eNOS) is usually expressed in endothelial cells and inducible nitric oxide synthase (iNOS) is mainly expressed in alveolar macrophages and epithelial cells. Both eNOS and iNOS are involved in lung inflammation. While they play several roles in lung inflammation formation and resolution, their expression and activity are also regulated by inflammatory factors. Their expression relationship in virus infection-induced lung injury is not well addressed. In this report, we analyzed expression of both eNOS and iNOS, the production of nitric oxide (NO) and reactive oxygen species (ROS), and expression of their associated regulatory proteins, heat shock protein 90 (HSP90) and caveolin-1 (Cav-1), in a swine lung injury model induced by porcine reproductive and respiratory syndrome virus (PRRSV) infection. The combination of upregulation of iNOS and downregulation of eNOS was observed in both natural and experimental PRRSV-infected lungs, while the combination is much enhanced in natural infected lungs. While NO production is much reduced in both infections, ROS was enhanced only in natural infected lungs. Moreover, HSP90 is increased in both natural and experimental infection and less Cav-1 expressed was observed only in the natural PRRSV-infected lungs. Therefore, the increased ROS generation is likely due to the increased iNOS and its unbalanced regulation by HSP90 and Cav-1, and it also likely causes higher endothelial dysfunction in clinical PRRSV-infected lungs.






Similar content being viewed by others
References
Felley-Bosco, Emanuela, Florent Bender, and Andrew F.G. Quest. 2002. Caveolin-1-mediated post-transcriptional regulation of inducible nitric oxide synthase in human colon carcinoma cells. Biological Research 35 (2): 169–176.
Förstermann, Ulrich, and Thomas Münzel. 2006. Endothelial nitric oxide synthase in vascular disease from marvel to menace. Circulation 113 (13): 1708–1714.
Forbes, Scott P, Ivan S Alferiev, Michael Chorny, Richard F Adamo, Robert J Levy, and Ilia Fishbein. 2013. Modulation of NO and ROS production by AdiNOS transduced vascular cells through supplementation with L-Arg and BH 4: Implications for gene therapy of restenosis. Atherosclerosis 230 (1):23–32.
Kleinert, Hartmut, Petra M Schwarz, and Ulrich Förstermann. 2003. Regulation of the expression of inducible nitric oxide synthase. Biological Chemistry 384 (10–11):1343–1364.
Jung, Won-Kyo, Inhak Choi, Da-Young Lee, Sung Su Yea, Yung Hyun Choi, Moon-Moo Kim, Sae-Gwang Park, Su-Kil Seo, Soo-Woong Lee, and Chang-Min Lee. 2008. Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW 264.7 macrophages via the p38/ERK and NF-κB pathways. The International Journal of Biochemistry & Cell Biology 40 (11): 2572–2582.
Balasubramaniam, Vivek, Anne M Maxey, Danielle B Morgan, Neil E Markham, and Steven H Abman. 2006. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. American Journal of Physiology-Lung Cellular and Molecular Physiology 291 (1):L119-L127.
Gross, Christine M, Ruslan Rafikov, Sanjiv Kumar, Saurabh Aggarwal, P Benson Ham III, Mary Louise Meadows, Mary Cherian-Shaw, Archana Kangath, Supriya Sridhar, and Rudolf Lucas. 2015. Endothelial nitric oxide synthase deficient mice are protected from lipopolysaccharide induced acute lung injury. PloS One 10 (3):e0119918.
Takenaka, Kaori, Yoshihiro Nishimura, Teruaki Nishiuma, Akihiro Sakashita, Tomoya Yamashita, Kazuyuki Kobayashi, Miyako Satouchi, Tatsuro Ishida, Seinosuke Kawashima, and Mitsuhiro Yokoyama. 2006. Ventilator-induced lung injury is reduced in transgenic mice that overexpress endothelial nitric oxide synthase. American Journal of Physiology-Lung Cellular and Molecular Physiology 290 (6): L1078–L1086.
Cuzzocrea, Salvatore, Emanuela Mazzon, Giusi Calabro, Laura Dugo, Angela De Sarro, Fons AJ van de Loo, and Achille P Caputi. 2000. Inducible nitric oxide synthase—knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. American Journal of Respiratory and Critical Care Medicine 162 (5):1859–1866.
Cuzzocrea, Salvatore, Emanuela Mazzon, Laura Dugo, Alberto Barbera, Tommaso Centorrino, Antonio Ciccolo, Maria Teresa Fonti, and Achille P Caputi. 2001. Inducible nitric oxide synthase knockout mice exhibit resistance to the multiple organ failure induced by zymosan. Shock 16 (1):51–58.
D’Alessio, Franco R, Kenji Tsushima, Neil R Aggarwal, Jason R Mock, Yoshiki Eto, Brian T Garibaldi, Daniel C Files, Claudia R Avalos, Jackie V Rodriguez, and Adam T Waickman. 2012. Resolution of experimental lung injury by monocyte-derived inducible nitric oxide synthase. The Journal of Immunology 189 (5):2234–2245.
Arriero, M.M., J.A. Rodriguez-Feo, A. Celdran, L. Sanchez de Miguel, F. Gonzalez-Fernandez, J. Fortes, A. Reyero, et al. 2000. Expression of endothelial nitric oxide synthase in human peritoneal tissue: regulation by Escherichia coli lipopolysaccharide. Journal of the American Society of Nephrology 11 (10): 1848–1856.
Drummond, Grant R, Hua Cai, Michael E Davis, Santhini Ramasamy, and David G Harrison. 2000. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circulation Research 86 (3):347–354.
Xi, Wang, Hiroko Satoh, Hiroyuki Kase, Kunihiro Suzuki, and Yoshiyuki Hattori. 2005. Stimulated HSP90 binding to eNOS and activation of the PI3–Akt pathway contribute to globular adiponectin-induced NO production: vasorelaxation in response to globular adiponectin. Biochemical and Biophysical Research Communications 332 (1):200–205.
Zhang, Yang, Xiang Li, Alexander Carpinteiro, Jeremy A Goettel, Matthias Soddemann, and Erich Gulbins. 2011. Kinase suppressor of Ras-1 protects against pulmonary Pseudomonas aeruginosa infections. Nature Medicine 17 (3):341–346.
Chen, Z., F.R. Bakhshi, A.N. Shajahan, T. Sharma, M. Mao, A. Trane, P. Bernatchez, et al. 2012. Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Molecular Biology of the Cell 23 (7): 1388–1398. doi:10.1091/mbc.E11-09-0811.
Cook, S., P. Vollenweider, B. Menard, M. Egli, P. Nicod, and U. Scherrer. 2003. Increased eNO and pulmonary iNOS expression in eNOS null mice. European Respiratory Journal 21 (5): 770–773.
Narasaraju, T., E. Yang, R.P. Samy, H.H. Ng, W.P. Poh, A.A. Liew, M.C. Phoon, N. van Rooijen, and V.T. Chow. 2011. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. American Journal of Pathology 179 (1): 199–210. doi:10.1016/j.ajpath.2011.03.013.
Brusselle, Guy G, Guy F Joos, and Ken R Bracke. 2011. New insights into the immunology of chronic obstructive pulmonary disease. The Lancet 378 (9795):1015–1026.
Qiao, Songlin, Lili Feng, Dengke Bao, Junqing Guo, Bo Wan, Zhijun Xiao, Suzhen Yang, and Gaiping Zhang. 2011. Porcine reproductive and respiratory syndrome virus and bacterial endotoxin act in synergy to amplify the inflammatory response of infected macrophages. Veterinary Microbiology 149 (1): 213–220.
Tian, Kegong, Xiuling Yu, Tiezhu Zhao, Youjun Feng, Zhen Cao, Chuanbin Wang, Yan Hu, Xizhao Chen, Dongmei Hu, and Xinsheng Tian. 2007. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PloS One 2 (6): e526.
Liu, Jie, Make Hou, Meiping Yan, Xinhui Lü, Gu Wei, Songlin Zhang, Jianfeng Gao, Bang Liu, Xiaoxiong Wu, and Guoquan Liu. 2015. ICAM-1-dependent and ICAM-1-independent neutrophil lung infiltration by porcine reproductive and respiratory syndrome virus infection. American Journal of Physiology-Lung Cellular and Molecular Physiology 309 (3): L226–L236.
Zhong, Weili, Guoliang Zou, Gu Jianqiu, and Jin Zhang. 2010. L-arginine attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells. Diabetes Research and Clinical Practice 89 (1): 38–45.
Peng, Yun-Li, Yu-Ning Liu, Lei Liu, Xia Wang, Chun-Lei Jiang, and Yun-Xia Wang. 2012. Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. Journal of Neuroinflammation 9 (1): 75.
Liu, Yuqiang, Jun He, Ling Jiang, Han Wu, Yuehua Xiao, Yanlin Liu, Guangquan Li, Du Yueqiang, Chenyang Liu, and Jianmin Wan. 2011. Nitric oxide production is associated with response to brown planthopper infestation in rice. Journal of Plant Physiology 168 (8): 739–745.
Fan, Jinli, Haibo Cai, and Wen-Song Tan. 2007. Role of the plasma membrane ROS-generating NADPH oxidase in CD34+ progenitor cells preservation by hypoxia. Journal of Biotechnology 130 (4): 455–462.
Zhao, Yuling, Naihao Lu, Hailing Li, Yan Zhang, Zhonghong Gao, and Yuefa Gong. 2008. High glucose induced human umbilical vein endothelial cell injury: Involvement of protein tyrosine nitration. Molecular and Cellular Biochemistry 311 (1–2): 19–29.
Guo, Chengzhi, Zuping He, Lixin Wen, Li Zhu, Yin Lu, Sijun Deng, Yang Yang, Qiang Wei, and Hui Yuan. 2012. Cytoprotective effect of trolox against oxidative damage and apoptosis in the NRK-52e cells induced by melamine. Cell Biology International 36 (2): 183–188.
Higashi, Yukihito, Kensuke Noma, Masao Yoshizumi, and Yasuki Kihara. 2009. Endothelial function and oxidative stress in cardiovascular diseases. Circulation Journal 73 (3): 411–418.
Sharma, Shruti, Anita Smith, Sanjiv Kumar, Saurabh Aggarwal, Imran Rehmani, Connie Snead, Cynthia Harmon, Jeffery Fineman, David Fulton, and John D Catravas. 2010. Mechanisms of nitric oxide synthase uncoupling in endotoxin-induced acute lung injury: role of asymmetric dimethylarginine. Vascular Pharmacology 52 (5):182–190.
Pautz, Andrea, Julia Art, Susanne Hahn, Sebastian Nowag, Cornelia Voss, and Hartmut Kleinert. 2010. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 23 (2): 75–93.
Förstermann, Ulrich, and William C Sessa. 2012. Nitric oxide synthases: regulation and function. European Heart Journal 33 (7):829–837.
Yoshida, Masako, and Yong Xia. 2003. Heat shock protein 90 as an endogenous protein enhancer of inducible nitric-oxide synthase. Journal of Biological Chemistry 278 (38): 36953–36958.
Winterbourn, Christine, Anthony J Kettle, and Mark B Hampton. 2016. Reactive oxygen species and neutrophil function. Annual Review of Biochemistry 85 (1).
Liu, G., A.T. Place, Z. Chen, V.M. Brovkovych, S.M. Vogel, W.A. Muller, R.A. Skidgel, A.B. Malik, and R.D. Minshall. 2012. ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood 120 (9): 1942–1952. doi:10.1182/blood-2011-12-397430.
Hoshiyama, Mari, Bing Li, Jian Yao, Takashi Harada, Tetsuo Morioka, and Takashi Oite. 2004. Effect of high glucose on nitric oxide production and endothelial nitric oxide synthase protein expression in human glomerular endothelial cells. Nephron Experimental Nephrology 95 (2): e62–e68.
Alp, Nicholas J, and Keith M Channon. 2004. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology 24 (3):413–420.
Mittal, Manish, Mohammad Rizwan Siddiqui, Khiem Tran, Sekhar P Reddy, and Asrar B Malik. 2014. Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling 20 (7):1126–1167.
Chen, Xin-xin, Rong Quan, Xue-kun Guo, Li Gao, Jishu Shi, and Wen-hai Feng. 2014. Up-regulation of pro-inflammatory factors by HP-PRRSV infection in microglia: implications for HP-PRRSV neuropathogenesis. Veterinary Microbiology 170 (1): 48–57.
Vlahos, R., J. Stambas, S. Bozinovski, B.R. Broughton, G.R. Drummond, and S. Selemidis. 2011. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathogens 7 (2): e1001271. doi:10.1371/journal.ppat.1001271.
Vlahos, Ross, John Stambas, and Stavros Selemidis. 2012. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends in Pharmacological Sciences 33 (1): 3–8.
Li, Yanchun, Darren F Boehning, Ting Qian, Vsevolod L Popov, and Steven A Weinman. 2007. Hepatitis C virus core protein increases mitochondrial ROS production by stimulation of Ca2+ uniporter activity. The FASEB Journal 21 (10):2474–2485.
Jin, S.W., C.Y. Choi, Y.P. Hwang, H.G. Kim, S.J. Kim, Y.C. Chung, K.J. Lee, T.C. Jeong, and H.G. Jeong. 2016. Betulinic acid increases eNOS phosphorylation and NO synthesis via the calcium-signaling pathway. Journal of Agricultural and Food Chemis ry 64 (4): 785–791. doi:10.1021/acs.jafc.5b05416.
Siragusa, M., F. Frohlich, E.J. Park, M. Schleicher, T.C. Walther, and W.C. Sessa. 2015. Stromal cell-derived factor 2 is critical for Hsp90-dependent eNOS activation. Science Signaling 8 (390): ra81. doi:10.1126/scisignal.aaa2819.
Schneider, J.C., D. El Kebir, C. Chereau, S. Lanone, X.L. Huang, A.S. De Buys Roessingh, J.C. Mercier, J. Dall’Ava-Santucci, and A.T. Dinh-Xuan. 2003. Involvement of Ca2+/calmodulin-dependent protein kinase II in endothelial NO production and endothelium-dependent relaxation. American Journal of Physiology Heart and Circulatory Physiology 284 (6): H2311–H2319. doi:10.1152/ajpheart.00932.2001.
Felley-Bosco, E., F. Bender, and A.F. Quest. 2002. Caveolin-1-mediated post-transcriptional regulation of inducible nitric oxide synthase in human colon carcinoma cells. Biological Research 35 (2): 169–176.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) Grants 31372418 (G. Liu) and 31201913 (S. Zhang), NSFC Major International Cooperation Project 31210103917 (B. Liu), Huazhong Agricultural University Scientific and Technology Self-Innovation Foundation Program 2012RC011 (G. Liu), and the 948 Project of Chinese Ministry of Agriculture (2015-Z33, GL).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Animal experiments were approved by Animal Care and Use Committee of Hubei Province, China, in accordance with guidelines developed by the China Council on Animal Care and protocol.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yan, M., Hou, M., Liu, J. et al. Regulation of iNOS-Derived ROS Generation by HSP90 and Cav-1 in Porcine Reproductive and Respiratory Syndrome Virus-Infected Swine Lung Injury. Inflammation 40, 1236–1244 (2017). https://doi.org/10.1007/s10753-017-0566-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0566-9