Abstract
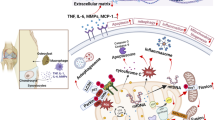
Inflammation and inflammatory cytokines have been reported to play vital roles in the development of osteoarthritis (OA). Plumbagin, a quinonoid compound extracted from the roots of medicinal herbs of the Plumbago genus, has been reported to have anti-inflammatory effects. However, the anti-inflammatory effects of plumbagin on OA have not been reported. This study aimed to assess the effects of plumbagin on human OA chondrocytes and in a mouse model of OA induced by destabilization of the medial meniscus (DMM). In vitro, human OA chondrocytes were pretreated with plumbagin (2, 5, 10 μM) for 2 h and subsequently stimulated with IL-1β for 24 h. Production of NO, PGE2, MMP-1, MMP-3, and MMP-13 was evaluated by the Griess reagent and ELISAs. The messenger RNA (mRNA) expression of COX-2, iNOS, MMP-1, MMP-3, MMP-13, aggrecan, and collagen-II was measured by real-time PCR. The protein expression of COX-2, iNOS, p65, p-p65, IκBα, and p-IκBα was detected by Western blot. The protein expression of collagen-II was evaluated by immunofluorescence. In vivo, the severity of OA was determined by histological analysis. We found that plumbagin significantly inhibited the IL-1β-induced production of NO and PGE2; expression of COX-2, iNOS, MMP-1, MMP-3, and MMP-13; and degradation of aggrecan and collagen-II. Furthermore, plumbagin dramatically suppressed IL-1β-stimulated NF-κB activation. In vivo, treatment of plumbagin not only prevented the destruction of cartilage and the thickening of subchondral bone but also relieved synovitis in mice OA models. Taken together, these results suggest that plumbagin may be a potential agent in the treatment of OA.







Similar content being viewed by others
Change history
04 March 2019
The original version of this article contained mistakes, and the authors would like to correct them. The correct details are given below:
References
Bitton, R. 2009. The economic burden of osteoarthritis. The American Journal of Managed Care 15: S230–235.
Loeser, R.F. 2009. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis and Cartilage 17: 971–979.
Niu, J., Y.Q. Zhang, J. Torner, M. Nevitt, C.E. Lewis, P. Aliabadi, et al. 2009. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis and Rheumatism 61: 329–335.
Lane, N.E., and M.C. Nevitt. 2002. Osteoarthritis, bone mass, and fractures: how are they related? Arthritis and Rheumatism 46: 1–4.
Bonnet, C.S., and D.A. Walsh. 2005. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 44: 7–16.
Abramson, S.B., M. Attur, A.R. Amin, and R. Clancy. 2001. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Current Rheumatology Reports 3: 535–541.
Kobayashi, M., G.R. Squires, A. Mousa, M. Tanzer, D.J. Zukor, J. Antoniou, et al. 2005. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis and Rheumatism 52: 128–135.
Eymard, F., A. Pigenet, D. Citadelle, C.H. Flouzat-Lachaniette, A. Poignard, C. Benelli, et al. 2014. Induction of an inflammatory and prodegradative phenotype in autologous fibroblast-like synoviocytes by the infrapatellar fat pad from patients with knee osteoarthritis. Arthritis & Rhematology 66: 2165–2174.
Chaganti, R.K., E. Purdue, T.P. Sculco, and L.A. Mandl. 2014. Elevation of serum tumor necrosis factor alpha in patients with periprosthetic osteolysis: a case–control study. Clinical Orthopaedics and Related Research 472: 584–589.
Tilak, J.C., S. Adhikari, and T.P. Devasagayam. 2004. Antioxidant properties of Plumbago zeylanica, an Indian medicinal plant and its active ingredient, plumbagin. Redox Report 9: 219–227.
Checker, R., D. Sharma, S.K. Sandur, S. Khanam, and T.B. Poduval. 2009. Anti-inflammatory effects of plumbagin are mediated by inhibition of NF-kappaB activation in lymphocytes. International Immunopharmacology 9: 949–958.
Ahmad, A., S. Banerjee, Z. Wang, D. Kong, and F.H. Sarkar. 2008. Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-kappaB and Bcl-2. Journal of Cellular Biochemistry 105: 1461–1471.
Mossa, J.S., F.S. El-Feraly, and I. Muhammad. 2004. Antimycobacterial constituents from Juniperus procera, Ferula communis and Plumbago zeylanica and their in vitro synergistic activity with isonicotinic acid hydrazide. Phytotherapy Research 18: 934–937.
Dzoyem, J.P., J.G. Tangmouo, D. Lontsi, F.X. Etoa, and P.J. Lohoue. 2007. In vitro antifungal activity of extract and plumbagin from the stem bark of Diospyros crassiflora Hiern (Ebenaceae). Phytotherapy Research 21: 671–674.
Wang, T., F. Wu, Z. Jin, Z. Zhai, Y. Wang, B. Tu, et al. 2014. Plumbagin inhibits LPS-induced inflammation through the inactivation of the nuclear factor-kappa B and mitogen activated protein kinase signaling pathways in RAW 264.7 cells. Food and Chemical Toxicology 64: 177–183.
Chu, H., H. Yu, D. Ren, K. Zhu, and H. Huang. 2016. Plumbagin exerts protective effects in nucleus pulposus cells by attenuating hydrogen peroxide-induced oxidative stress, inflammation and apoptosis through NF-kappaB and Nrf-2. International Journal of Molecular Medicine 37: 1669–1676.
Checker, R., R.S. Patwardhan, D. Sharma, J. Menon, M. Thoh, S.K. Sandur, et al. 2014. Plumbagin, a vitamin K3 analogue, abrogates lipopolysaccharide-induced oxidative stress, inflammation and endotoxic shock via NF-kappaB suppression. Inflammation 37: 542–554.
Luo, P., Y.F. Wong, L. Ge, Z.F. Zhang, Y. Liu, L. Liu, et al. 2010. Anti-inflammatory and analgesic effect of plumbagin through inhibition of nuclear factor-kappaB activation. The Journal of Pharmacology and Experimental Therapeutics 335: 735–742.
Palmieri, B., D. Lodi, and S. Capone. 2010. Osteoarthritis and degenerative joint disease: local treatment options update. Acta Biomed 81: 94–100.
Au, R.Y., T.K. Al-Talib, A.Y. Au, P.V. Phan, and C.G. Frondoza. 2007. Avocado soybean unsaponifiables (ASU) suppress TNF-alpha, IL-1beta, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthritis and Cartilage 15: 1249–1255.
Pfaffl, M.W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29: e45.
Vasheghani, F., Y. Zhang, Y.H. Li, M. Blati, H. Fahmi, B. Lussier, et al. 2015. PPARgamma deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage. Annals of the Rheumatic Diseases 74: 569–578.
Pritzker, K.P., S. Gay, S.A. Jimenez, K. Ostergaard, J.P. Pelletier, P.A. Revell, et al. 2006. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis and Cartilage 14: 13–29.
Lewis, J.S., W.C. Hembree, B.D. Furman, L. Tippets, D. Cattel, J.L. Huebner, et al. 2011. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis and Cartilage 19: 864–873.
Bonassar, L.J., J.D. Sandy, M.W. Lark, A.H. Plaas, E.H. Frank, and A.J. Grodzinsky. 1997. Inhibition of cartilage degradation and changes in physical properties induced by IL-1beta and retinoic acid using matrix metalloproteinase inhibitors. Archives of Biochemistry and Biophysics 344: 404–412.
Sasaki, K., T. Hattori, T. Fujisawa, K. Takahashi, H. Inoue, and M. Takigawa. 1998. Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. Journal of Biochemistry 123: 431–439.
Goggs, R., S.D. Carter, G. Schulze-Tanzil, M. Shakibaei, and A. Mobasheri. 2003. Apoptosis and the loss of chondrocyte survival signals contribute to articular cartilage degradation in osteoarthritis. The Veterinary Journal 166: 140–158.
Li, N., M.A. Rivera-Bermudez, M. Zhang, J. Tejada, S.S. Glasson, L.A. Collins-Racie, et al. 2010. LXR modulation blocks prostaglandin E2 production and matrix degradation in cartilage and alleviates pain in a rat osteoarthritis model. Proceedings of the National Academy of Sciences of the United States of America 107: 3734–3739.
Wang, Y., L. Li de, X.B. Zhang, Y.H. Duan, Z.H. Wu, D.S. Hao, et al. 2013. Increase of TNFalpha-stimulated osteoarthritic chondrocytes apoptosis and decrease of matrix metalloproteinases 9 by NF-kappaB inhibition. Biomedical and Environmental Sciences 26: 277–283.
Brinckerhoff, C.E., and L.M. Matrisian. 2002. Matrix metalloproteinases: a tail of a frog that became a prince. Nature Reviews Molecular Cell Biology 3: 207–214.
Tetlow, L.C., D.J. Adlam, and D.E. Woolley. 2001. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis and Rheumatism 44: 585–594.
Yoshihara, Y., H. Nakamura, K. Obata, H. Yamada, T. Hayakawa, K. Fujikawa, et al. 2000. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Annals of the Rheumatic Diseases 59: 455–461.
So, J.S., M.K. Song, H.K. Kwon, C.G. Lee, C.S. Chae, A. Sahoo, et al. 2011. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sciences 88: 358–366.
Suh, H.J., H. Lee, B.J. Min, S.U. Jung, and E.Y. Jung. 2016. Effects of gangliosides from deer bone extract on the gene expressions of matrix metalloproteinases and collagen type II in interleukin-1beta-induced osteoarthritic chondrocytes. Nutrition for Research and Practice 10: 569–574.
Oeckinghaus, A., and S. Ghosh. 2009. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harbor Perspectives in Biology 1: a000034.
Rigoglou, S., and A.G. Papavassiliou. 2013. The NF-kappaB signalling pathway in osteoarthritis. The International Journal of Biochemistry & Cell Biology 45: 2580–2584.
Roman-Blas, J.A., and S.A. Jimenez. 2006. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis and Cartilage 14: 839–848.
Wang, S.N., G.P. Xie, C.H. Qin, Y.R. Chen, K.R. Zhang, X. Li, et al. 2015. Aucubin prevents interleukin-1 beta induced inflammation and cartilage matrix degradation via inhibition of NF-kappaB signaling pathway in rat articular chondrocytes. International Immunopharmacology 24: 408–415.
Liacini, A., J. Sylvester, W.Q. Li, and M. Zafarullah. 2002. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biology 21: 251–262.
Lianxu, C., J. Hongti, and Y. Changlong. 2006. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthritis and Cartilage 14: 367–376.
Jia, Y., J. Jing, Y. Bai, Z. Li, L. Liu, J. Luo, et al. 2011. Amelioration of experimental autoimmune encephalomyelitis by plumbagin through down-regulation of JAK-STAT and NF-kappaB signaling pathways. PloS One 6: e27006.
Zheng, W., H. Zhang, Y. Jin, Q. Wang, L. Chen, Z. Feng, et al. 2016. Butein inhibits IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes and slows the progression of osteoarthritis in mice. International Immunopharmacology 42: 1–10.
Wei, Y., and L. Bai. 2016. Recent advances in the understanding of molecular mechanisms of cartilage degeneration, synovitis and subchondral bone changes in osteoarthritis. Connective Tissue Research 57: 245–261.
Acknowledgments
The authors thank all the staff in the Laboratory of Orthopaedic Research Institute and Scientific Research Center of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University. This work was supported by grants from the National Natural Science Foundation of China (81402980).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was in accordance with the Declaration of Helsinki and Tokyo.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zheng, W., Tao, Z., Chen, C. et al. Plumbagin Prevents IL-1β-Induced Inflammatory Response in Human Osteoarthritis Chondrocytes and Prevents the Progression of Osteoarthritis in Mice. Inflammation 40, 849–860 (2017). https://doi.org/10.1007/s10753-017-0530-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0530-8