Abstract
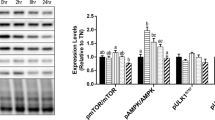
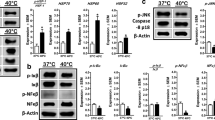
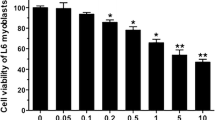
This study explored that lipoic acid treatment for 24 h significantly upregulated and promoted heat shock-induced catalase expression and downregulated GPx1 messenger RNA (mRNA) expression, indicating that lipoic acid exhibits antioxidant activity in the decomposition of hydrogen peroxide by upregulating catalase expression. Moreover, lipoic acid treatment for 3 h increased and promoted heat shock-induced interleukin (IL)-6 mRNA and protein levels and that for 24 h downregulated IL-6 mRNA expression, suggesting a dual effect of lipoic acid on IL-6 regulation. Lipoic acid alone failed to increase or reduce tumor necrosis factor (TNF)-α mRNA and protein levels, whereas heat shock alone downregulated TNF-α mRNA and protein expression. These data suggest that lipoic acid does not have a proinflammatory role and that heat shock acts as an anti-inflammatory agent by downregulating TNF-α expression in C2C12 myotubes. Moreover, lipoic acid or heat shock alone upregulated the IL-6 receptor (IL-6R-α) and glycoprotein 130 (gp130) mRNA expression followed by IL-6 expression; these data indicate that the regulation of lipoic acid or heat shock is mediated by IL-6R signaling, thus suggesting that C2C12 myotubes possesses a mechanism for regulating IL-6R and gp130 expression following lipoic acid treatment or heat shock.




Similar content being viewed by others
REFERENCES
Akerfelt, M., R.I. Morimoto, and L. Sistonen. 2010. Heat shock factors: integrators of cell stress, development and lifespan. Nature Reviews Molecular Cell Biology 11: 545–555.
Demarco, V.G., P.O. Scumpia, J.P. Bosanquet, and J.W. Skimming. 2004. Alpha-lipoic acid inhibits endotoxin-stimulated expression of iNOS and nitric oxide independent of the heat shock response in RAW 264.7 cells. Free Radical Research 38: 675–682.
Tyagi, N., and R. Tyagi. 2015. The wonderous chaperones: a highlight on therapeutics of cancer and potentially malignant disorders. Journal of Oral and Maxillofacial Pathology 19: 212–220.
Wieten, L., F. Broere, R. van der Zee, E.K. Koerkamp, J. Wagenaar, and W. van Eden. 2007. Cell stress induced HSP are targets of regulatory T cells: a role for HSP inducing compounds as anti-inflammatory immuno-modulators? FEBS Letters 581: 3716–3722.
Kim, I., H.M. Shin, and W. Baek. 2005. Heat-shock response is associated with decreased production of interleukin-6 in murine aortic vascular smooth muscle cells. Naunyn-Schmiedeberg's Archives of Pharmacology 371: 27–33.
Pockley, A.G., S.K. Calderwood, and G. Multhoff. 2009. The atheroprotective properties of Hsp70: a role for Hsp70-endothelial interactions? Cell Stress & Chaperones 14: 545–553.
Bast, A., and G.R. Haenen. 1988. Interplay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochimica et Biophysica Acta 963: 558–561.
Maczurek, A., K. Hager, M. Kenklies, M. Sharman, R. Martins, J. Enge, D.A. Carlson, and G. Münch. 2008. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer's disease. Advanced Drug Delivery Reviews 60: 1463–1470.
Petronilho, F., D. Florentino, L.G. Danielski, L.C. Vieira, M.M. Martins, A. Vieira, S. Bonfante, M.P. Goldim, and F. Vuolo. 2015. Alpha-Lipoic Acid Attenuates Oxidative Damage in Organs After Sepsis. Inflammation Oct 2.
Gorąca, A., H. Huk-Kolega, A. Piechota, P. Kleniewska, E. Ciejka, and B. Skibska. 2011. Lipoic acid—biological activity and therapeutic potential. Pharmacological Reports 63: 849–858.
Gianturco, V., A. Bellomo, E. D'Ottavio, V. Formosa, A. Iori, M. Mancinella, G. Troisi, and V. Marigliano. 2009. Impact of therapy with alpha-lipoic acid (ALA) on the oxidative stress in the controlled NIDDM: a possible preventive way against the organ dysfunction? Archives of Gerontology and Geriatrics 49(Suppl 1): 129–133.
Kiemer, A.K., C. Müller, and A.M. Vollmar. 2002. Inhibition of LPS-induced nitric oxide and TNF-alpha production by alpha-lipoic acid in rat Kupffer cells and in RAW 264.7 murine macrophages. Immunology and Cell Biology 80: 550–557.
Zhang, W.J., and B. Frei. 2001. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. The FASEB Journal 15: 2423–2432.
Wong, A., S. Dukic-Stefanovic, J. Gasic-Milenkovic, R. Schinzel, H. Wiesinger, P. Riederer, and G. Münch. 2001. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. European Journal of Neuroscience 14: 1961–1967.
Bierhaus, A., S. Chevion, M. Chevion, M. Hofmann, P. Quehenberger, T. Illmer, T. Luther, E. Berentshtein, H. Tritschler, M. Müller, P. Wahl, R. Ziegler, and P.P. Nawroth. 1997. Advanced glycation end product-induced activation of NF-kappaB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes 46: 1481–1490.
Wollin, S.D., and P.J.H. Jones. 2003. α-lipoic acid and cardiovascular disease. Journal of Nutrition 133: 3327–3330.
Xiao, Z.Q., L. Moragoda, R. Jaszewski, J.A. Hatfield, S.E.G. Fligiel, and A.P.N. Majumdar. 2001. Aging is associated with increased proliferation and decreased apoptosis in the colonic mucosa. Mechanisms of Ageing and Development 122: 1849–1864.
Bertolotto, F., and A. Massone. 2012. Combination of alpha lipoic acid and superoxide dismutase leads to physiological and symptomatic improvements in diabetic neuropathy. Drugs in R&D 12: 29–34.
Hirano, T. 1998. Interleukin 6 and its receptor: ten years later. International Reviews of Immunology 16: 249–284.
Conti, B., I. Tabarean, C. Andrei, and T. Bartfai. 2004. Cytokines and fever. Frontiers in Bioscience 9: 1433–1449.
Nagaraju, K., N. Raben, G. Merritt, L. Loeffler, K. Kirk, and P. Plotz. 1998. A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli. Clinical and Experimental Immunology 113: 407–414.
Ostrowski, K., T. Rohde, M. Zacho, S. Asp, and B.K. Pedersen. 1998. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. The Journal of Physiology 508: 949–953.
Kosmidou, I., T. Vassilakopoulos, A. Xagorari, S. Zakynthinos, A. Papapetropoulos, and C. Roussos. 2002. Production of interleukin-6 by skeletal myotubes: role of reactive oxygen species. American Journal of Respiratory Cell and Molecular Biology 26: 587–593.
Welc, S.S., N.A. Phillips, J. Oca-Cossio, S.M. Wallet, D.L. Chen, and T.L. Clanton. 2012. Hyperthermia increases interleukin-6 in mouse skeletal muscle. American Journal of Physiology - Cell Physiology 303: C455–466.
Welc, S.S., A.R. Judge, and T.L. Clanton. 2013. Skeletal muscle interleukin-6 regulation in hyperthermia. American Journal of Physiology - Cell Physiology 305: C406–413.
Pedersen, B.K., and M.A. Febbraio. 2008. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiological Reviews 88: 1379–1406.
Portier, G.L., A.G. Benders, A. Oosterhof, J.H. Veerkamp, and T.H. van Kuppevelt. 1999. Differentiation markers of mouse C2C12 and rat L6 myogenic cell lines and the effect of the differentiation medium. In Vitro Cellular and Developmental Biology Animal 35: 219–227.
Wu, P.F., S.C. Luo, and L.C. Chang. 2015. Heat-shock-induced glucose transporter 4 in the slow-twitch muscle of rats. Physiological Research 64: 523–530.
Fridovich, I. 1995. Superoxide radical and superoxide dismutases. Annual Review of Biochemistry 64: 97–112.
Kirkman, H.N., and G.F. Gaetani. 2007. Mammalian catalase: a venerable enzyme with new mysteries. Trends in Biochemical Sciences 32: 44–50.
Chada, S., C. Whitney, and P.E. Newburger. 1989. Post-transcriptional regulation of glutathione peroxidase gene expression by selenium in the HL-60 human myeloid cell line. Blood 74: 2535–2541.
Mihara, M., M. Hashizume, H. Yoshida, M. Suzuki, and M. Shiina. 2012. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clinical Science (London, England : 1979) 122: 143–159.
Rose-John, S. 2012. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. International Journal of Biological Sciences 8: 1237–1247.
Muñoz-Cánoves, P., C. Scheele, B.K. Pedersen, and A.L. Serrano. 2013. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS Journal 280: 4131–4148.
Kostek, M.C., K. Nagaraju, E. Pistilli, A. Sali, S.H. Lai, B. Gordon, and Y.W. Chen. 2012. IL-6 signaling blockade increases inflammation but does not affect muscle function in the mdx mouse. BMC Musculoskeletal Disorders 13: 106.
Starkie, R., S.R. Ostrowski, S. Jauffred, M. Febbraio, and B.K. Pedersen. 2003. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. The FASEB Journal 17: 884–886.
Jones, S.A., J. Scheller, and S. Rose-John. 2011. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. The Journal of Clinical Investigation 121: 3375–3383.
Kishimoto, T. 2010. IL-6: from its discovery to clinical applications. International Immunology 22: 347–352.
Burton, M.D., N.L. Sparkman, and R.W. Johnson. 2011. Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. Journal of Neuroinflammation 8: 54.
Campbell, I.L., M. Erta, S.L. Lim, R. Frausto, U. May, S. Rose-John, J. Scheller, and J. Hidalgo. 2014. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. The Journal of Neuroscience 34: 2503–2513.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by a grant from Zuoying Branch of Kaohsiung Armed Forces General Hospital, Kaohsiung, Taiwan (ZBH104-14).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lee, CT., Chang, LC. & Wu, PF. Lipoic Acid Exerts Antioxidant and Anti-inflammatory Effects in Response to Heat Shock in C2C12 Myotubes. Inflammation 39, 1160–1168 (2016). https://doi.org/10.1007/s10753-016-0350-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0350-2