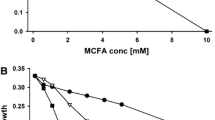
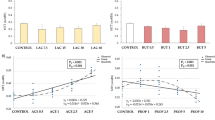
Abstract
Reactive oxygen species are implicated in cell and tissue damage in a number of diseases including acute and chronic inflammation of the gut. Effects of H2O2 exposure on non-carcinogenic porcine epithelial cell line, IPEC-J2 cells cultured on collagen-coated membrane inserts were monitored based on transepithelial electrical resistance (TER) change, extent of necrotic cell damage, gene expression of inflammatory cytokines IL-8 and TNF-α. Furthermore, the junction proteins claudin-1 and E-cadherin were also investigated by immunohistochemistry. Peroxide (1mM) increased IL-8 and TNF-α gene expression levels significantly allowing 1 h recovery time without affecting the cellular distribution of junction proteins, TER and cell survival rate. In conclusion, the IPEC-J2 cell line on membrane insert was introduced as a fast and reliable investigation tool for oxidative stimuli-triggered intestinal inflammation and in the future as a screening method for antioxidant and probiotic candidates.






Similar content being viewed by others
References
Rao, R.K., R.D. Baker, S.S. Baker, A. Gupta, and M. Holycross. 1997. Oxidant-induced disruption of intestinal epithelial barrier function: Role of protein tyrosine phosphorylation. AJP-Gastrointestinal and Liver Physiology 273: G812–G823.
Meyer, T.N., C. Schwesinger, J. Ye, B.M. Denker, and S.K. Nigam. 2001. Reassembly of the tight junction after oxidative stress depends on tyrosinase kinase activity. Journal of Biological Chemistry 276: 22048–22055.
Son, D.O., H. Satsu, and M. Shimizu. 2005. Histidine inhibits oxidative stress- and TNF-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Letters 579: 4671–4677.
Yamamoto, K., R. Kushima, O. Kisaki, Y. Fujiyama, and H. Okabe. 2003. Combined effect of hydrogen peroxide induced oxidative stress and IL-1alpha on IL-8 production in CaCo-2 cells (a human colon carcinoma cell line) and normal intestinal epithelial cells. Inflammation 27: 123–128.
Shimada, T., N. Watanabe, H. Hiraishi, and A. Terano. 1999. Redox regulation of interleukin-8 expression in MKN 28 cells. Digestive Diseases and Sciences 44: 266–273.
Sen, C.K., and L. Packer. 1996. Antioxidant and redox regulation of gene transcription. The FASEB Journal 10: 709–720.
Sun, Y., and L.W. Oberley. 1996. Redox regulation of transcriptional activators. Free Radical Biology & Medicine 21: 335–348.
Berschneider, H.M. 1989. Development of normal cultured small intestinal epithelial cell lines which transport Na and Cl. Gastroenterology 96: A41.
Cencic, A., and T. Langerholc. 2010. Functional cell models of the gut and their applications in food microbiology—A review. International Journal of Food Microbiology 141: S4–S14.
Schierack, P., M. Nordhoff, M. Pollmann, K.D. Weyrauch, S. Amasheh, U. Lodemann, J. Jores, B. Tachu, S. Kleta, A. Blikslager, K. Tedin, and L.H. Wieler. 2006. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochemistry and Cell Biology 125: 293–305.
González-Mariscal, L., A. Betanzos, P. Nava, and B.E. Jaramillo. 2003. Tight junction proteins. Progress in Biophysics and Molecular Biology 81: 1–44.
Oliveira, S.S., and J.A. Morgado-Diaz. 2007. Claudins: Multifunctional players in epithelial tight junctions and their role in cancer. Cellular and Molecular Life Science 64: 17–28.
Reyes, J.L., M. Lamas, D. Martin, M. del Carmen Namorado, S. Islas, J. Luna, M. Tauc, and L. González-Mariscal. 2002. The renal segmental distribution of claudins changes with development. Kidney International 62: 476–487.
Inai, T., J. Kobayashi, and Y. Shibata. 1999. Claudin-1 contributes to the epithelial barrier function in MDCK cells. European Journal of Cell Biology 78: 849–855.
McCarthy, K.M., S.A. Francis, J.M. McCormack, J. Lai, R.A. Rogers, I.B. Skare, R.D. Lynch, and E.E. Schneeberger. 2000. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. Journal of Cell Science 113: 3387–3398.
Sonoda, N., M. Furuse, H. Sasaki, S. Yonemura, J. Katahira, Y. Horiguchi, and S. Tsukita. 1999. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. The Journal of Cell Biology 147: 195–204.
Hou, J., A.S. Gomes, D.L. Paul, and D.A. Goodenough. 2006. Study of claudin function by RNA interference. Journal of Biological Chemistry 281: 36117–36123.
Oshima, T., M. Sasaki, H. Kataoka, H. Miwa, T. Takeuchi, and T. Joh. 2007. Wip1 protects hydrogen peroxide-induced colonic epithelial barrier dysfunction. Cellular and Molecular Life Science 64: 3139–3147.
Park, J.Y., K.H. Park, T.Y. Oh, S.P. Hong, T.J. Jeon, C.H. Kim, S.W. Park, J.B. Chung, S.Y. Song, and S. Bang. 2007. Up-regulated claudin 7 expression in intestinal-type gastric carcinoma. Oncology Reports 18: 377–382.
Van Itallie, C., C. Rahner, and J.M. Anderson. 2001. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. Journal of Clinical Investigation 107: 1319–1327.
Hashimoto, K., T. Oshima, T. Tomita, Y. Kim, T. Matsumoto, T. Joh, and H. Miwa. 2008. Oxidative stress induces gastric epithelial permeability through claudin-3. Biochemical and Biophysical Research Communications 376: 154–157.
Nowak, D. 1990. Hydrogen peroxide release from human polymorphonuclear leukocytes measured with horseradish peroxidase and o-dianisidine. Effect of various stimulators and cytochalasin B. Biomedica Biochimica Acta 49: 353–362.
Sakumoto, R., T. Komatsu, E. Kasuya, T. Saito, and K. Okuda. 2006. Expression of mRNAs for interleukin-4, interleukin-6 and their receptors in porcine corpus luteum during the estrous cycle. Domestic Animal Endocrinology 31: 246–257.
Hyland, K.A., D.R. Brown, and M.P. Murtaugh. 2006. Salmonella enterica serovar Choleraesuis infection of the porcine jejunal Peyer's patch rapidly induces IL-1beta and IL-8 expression. Veterinary Immunology and Immunpathology 109: 1–11.
Nygard, A.B., C.B. Jorgensen, S. Cirera, and M. Fredholm. 2007. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Molecular Biology 8: 67.
Nemeth, E., A. Halasz, A. Barath, M. Domokos, and P. Galfi. 2007. Effect of hydrogen peroxide on interleukin-8 synthesis and death of Caco-2 cells. Immunpharmacology and Immuntoxicology 29: 297–310.
Davies, K.J. 1995. Oxidative stress: The paradox of aerobic life. Biochemical Society Symposia 61: 1–31.
Finkel, T., and N.J. Holbrook. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408(6809): 239–247.
Schroder, P., and J. Krutmann. 2005. Environmental oxidative stress—Environmental sources of ROS. In: The Handbook of Environmental Chemistry, Springer-Verlag, Berlin/Heidelberg, pp. 19–31
Harris, M.L., H.J. Schiller, P.M. Reilly, M. Donowitz, M.B. Grisham, and G.B. Bulkley. 1992. Free radicals and other reactive oxygen metabolites in inflammatory bowel disease: Cause, consequence or epiphenomenon? Pharmacology and Therapeutics 53: 375–408.
Blau, S., A. Rubinstein, P. Bass, C. Singaram, and R. Kohen. 1999. Differences in the reducing power along the rat GI tract: Lower antioxidant capacity of the colon. Molecular and Cellular Biochemistry 194: 185–191.
Giandomenico, A.R., G.E. Cerniglia, J.E. Biaglow, C.W. Stevens, and C.J. Koch. 1997. The importance of sodium pyruvate in assessing damage produced by hydrogen peroxide. Free Radical Biology & Medicine 23: 426–434.
Jacewicz, D., M. Szkatula, A. Chylewska, A. Dabrowska, M. Wozniak, and L. Chmurzynsk. 2008. Coordinate cis-[Cr(C2O4)(pm)(OH2)2]+ cation as molecular biosensor of pyruvate’s protective activity against hydrogen peroxide mediated cytotoxity. Sensors 8: 4487–4504.
Bienert, G.P., J.K. Schjoerring, and T.P. Jahn. 2006. Membrane transport of hydrogen peroxide. Biochimica et Biophysica Acta 1758: 994–1003.
Antunes, F., and E. Cadenas. 2000. Estimation of H2O2 gradients across biomembranes. FEBS Letters 475: 121–126.
Valko, M., D. Leibfritz, J. Moncol, M.T. Cronin, M. Mazur, and J. Telser. 2007. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology 39: 44–84.
Saberi, B., M. Shinohara, M.D. Ybanez, N. Hanawa, W.A. Gaarde, N. Kaplowitz, and D. Han. 2008. Regulation of H2O2-induced necrosis by PKC and AMP-activated kinase signaling in primary cultured hepatocytes. AJP-Cell Physiology 295: C50–C63.
Sheth, P., S. Basuroy, C. Li, A.P. Naren, and R.K. Rao. 2003. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. Journal of Biological Chemistry 278: 49239–49245.
Seth, A., F. Yan, D.B. Polk, and R.K. Rao. 2008. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. AJP-Gastrointestinal and Liver Physiology 294: G1060–G1069.
Miyauchi, E., H. Morita, and S. Tanabe. 2009. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. Journal of Diary Science 92: 2400–2408.
Qin, H., Z. Zhang, X. Hang, and Y. Jiang. 2009. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiology 9: 63.
Mennigen, R., K. Nolte, E. Rijcken, M. Utech, B. Loeffler, N. Senninger, and M. Bruewer. 2009. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. AJP-Gastrointestinal and Liver Physiology 296: G1140–G1149.
Stadnyk, A.W. 1994. Cytokine production by epithelial cells. The FASEB Journal 8: 1041–1047.
Dinarello, C.A. 2000. Proinflammatory cytokines. Chest 118: 503–508.
Walsh, S.V., A.M. Hopkins, and A. Nusrat. 2000. Modulation of tight junction structure and function by cytokines. Advanced Drug Delivery Reviews 41: 303–313.
Ma, T.Y., J.K. Ivamoto, N.T. Hoa, V. Akotia, A. Pedram, M.A. Boivin, and H.M. Said. 2004. TNF-alpha- increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. AJP-Gastrointestinal and Liver Physiology 286: G364–G376.
Ye, D., I. Ma, and T.Y. Ma. 2006. Molecular mechanism of tumor necrosis factor alpha modulation of intestinal epithelial tight junction barrier. AJP-Gastrointestinal and Liver Physiology 290: G496–G504.
Tabibzadeh, S., Q.F. Kong, S. Kapur, P.G. Satyaswaroop, and K. Aktories. 1995. Tumor necrosis factor-alpha-mediated dyscohesion of epithelial cells is associated with disordered expression of cadherin/beta-catenin and disassembly of actin filaments. Human Reproduction 10: 994–1004.
Schmitz, H., M. Fromm, C.J. Bentzel, P. Scholz, K. Detjen, J. Mankertz, H. Bode, H.J. Epple, E.O. Riecken, and J.D. Schulzke. 1999. TNF alpha regulates the epithelial barrier in the human intestinal cell line HT-29/B6. Journal of Cell Science 112: 137–146.
Rollo, E.E., S.J. Hempson, A. Bansal, E. Tsao, I. Habib, S.R. Ritting, D.T. Denhardt, E.R. Mackow, and R.D. Shaw. 2005. The cytokine osteopontin modulates the severity of rotavirus diarrhoea. Journal of Virology 79: 3509–3516.
Johnson, A.M., R.S. Kaushik, and P.R. Hardwidge. 2010. Disruption of transepithelial resistance by enterotoxigenic Escherichia coli. Veterinary Microbiology 141: 115–119.
Acknowledgement
The research described here has been supported by the Hungarian Scientific Research Fund (grant OTKA no. 76133). We are indebted to Dr. Jody Gookin and Dr. Stephen Stauffer, Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC, USA, for providing IPEC-J2 cells and for the valuable advice on handling them. We also would to thank Dr. Adam Csordas, Division of Medical Biochemistry, Biocenter, Innbruck Medical University, Innsbruck, Austria, for critical reading of the manuscript. Besides useful practical and theoretical guidance, support in sequencing of PCR product (IL-8) from Dr. Balazs Gereben, Department of Endocrine Neurobiology, Institute of Experimental Medicine of the Hungarian Academy of Sciences, is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paszti-Gere, E., Csibrik-Nemeth, E., Szeker, K. et al. Acute Oxidative Stress Affects IL-8 and TNF-α Expression in IPEC-J2 Porcine Epithelial Cells. Inflammation 35, 994–1004 (2012). https://doi.org/10.1007/s10753-011-9403-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-011-9403-8