Abstract
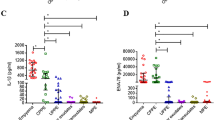
Intrapleural fibrinolytic therapy (IFT) provides clinical benefit in the treatment of complicated pleural parapneumonic effusion (CPE). Whether IFT influences the proinflammatory cytokines production and fibrinlytic activity is currently unclear. Therefore, we collected pleural effusion samples from CPE patients with IFT (study group) and patients without IFT (control group). A membrane human inflammatory cytokines array kit was used to compare the difference of targeted cytokine production between these two groups. Enzyme-linked immunosorbent assay (ELISA) methods were used for quantitative analysis of targeted cytokines and fibrinolytic enzymes. The results showed there were no significant differences between the study (n = 16) and control (n = 14) groups in patients’ demographic data. After fibrinolytic therapy, the patients in the study group had significant lower plasminogen activator inhibitor (PAI) level (732.36 ± 254.09 ng/mL vs 1,509.36 ± 1,340.11 ng/mL, p < 0.05) and higher urokinase plasminogen activator (u-PA) level (75.56 ± 41.70 ng/mL vs 6.87 ± 5.07 ng/mL, p < 0.05) than they did before treatment. Moreover, the tissue inhibitors of metalloproteinase-2 (TIMP-2) (1,560.03 ± 403.49 pg/mL vs 3,686.45 ± 1,263.83 pg/mL, p < 0.05) and inflammatory chemokine, regulated on activation normal T-cell expressed and secreted/chemokine (C-C motif) ligand 5 (RANTES), (293.58 ± 212.93 pg/mL vs 749.27 ± 53.79 pg/mL, p < 0.05), were also significantly lower in the study group after fibrinolytic therapy, but not in the control group. In conclusion, intrapleural fibrinolytic treatment with urokinase could enhance fibrinolytic activity and decrease TIMP-2 and RANTES production.


Similar content being viewed by others
References
Idell, S., C. Zwieb, A. Kumar, K. B. Koenig, and A. R. Johnson. 1992. Pathways of fibrin turnover of human pleural mesothelial cells in vitro. Am. J. Respir. Cell. Mol. Biol. 7:414–426.
Agrenius, V., J. Chmielewska, O. Widström, and M. Blombäck. 1989. Pleural fibrinolytic activity is decreased in inflammation as demonstrated in quinacrine pleurodesis treatment of malignant pleural effusion. Am. Rev. Respir. Dis. 140:1381–1385.
Hua, C. C., L. C. Chang, Y. C. Chen, and S. C. Chang. 1999. Proinflammatory cytokines and fibrinolytic enzymes in tuberculous and malignant pleural effusions. Chest 116:1292–1296.
Krishnan, S., N. Amin, A. J. Dozor, and G. Stringel. 1997. Urokinase in the management of complicated parapneumonic effusions in children. Chest 112:1579–1583.
Bithell, T. C. 1993. Blood coagulation. In: Wintrobe’s Clinical Hematology, 9th edn., Lee, Bithell, Foerster, eds. Philadelphia, Lea & Febiger.
Whawell, S. A., and J. N. Thompson. 1995. Cytokine-induced release of plasminogen activator inhibitor-1 by human mesothelial cells. Eur. J. Surg. 161:315–317.
Philip-Joët, F., M. C. Alessi, C. Philip-Joët, M. Aillaud, J. R. Barriere, A. Arnaud, and I. Juhan-Vague. 1995. Fibrinolytic and inflammatory processes in pleural effusions. Eur. Respir. J. 8:1352–1356.
Chung, C. L., C. H. Chen, J. R. Sheu, Y. C. Chen, and S. C. Chang. 2005. Proinflammatory cytokines, transforming growth factor-ß1, and fibrinolytic enzymes in loculated and free-flowing pleural exudates. Chest 128:690–697.
Tillett, W. S., and S. Sherry. 1949. The effect in patients with streptococcal fibrinolysis (streptokinasse) and streptococcal desoxyribonuclease on fibrinous, purulent, and sanguinous pleural exudations. J. Clin. Invest. 28:173–190.
Thomson, A. H., J. Hull, M. R. Kumar ,, C. Wallis, and I. M. Balfour Lynn. 2002. Randomised trial of intrapleural urokinase in the treatment of childhood empyema. Thorax 57:343–347.
Barbato, A., C. Panizzolo, C. Monciotti, F. Marcucci, G. Stefanutti, and P. G. Gamb Gamba. 2003. Use of urokinase in childhood pleural empyema. Pediatr. Pulmonol. 35:50–55.
Marder, V. J., and S. Sherry. 1988. Thrombolytic therapy: current status (1). N. Eng. J. Med. 318:1512–1520.
Bouros, D., S. Schiza, G. Patsourakis, G. Chalkiadakis, P. Panagou, and N. Siafakas. 1997. Intrapleural streptokinasse versus normal saline in the treatment of complicated parapneumonic effusions and empyema. Am. J. Respir. Crit. Care Med. 155:291–295.
Davies, R. J. O., Z. C. Traill, and F. V. Gleeson. 1997. Randomised controlled trial of intrapleureal streptokinase in community acquired pleural infection. Thorax 52:416–421.
Cameron, R. J., and H. R. Davies. 2008. Intra-pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Sys. Rev. (2): Art. No. CD002312. doi:10.1002/14651858.
Yao, C. T., J. M. Wu, C. C. Liu, M. H. Wu, H. Y. Chuang, and J. N. Wang. 2004. Treatment of complicated parapneumonic pleural effusion with intrapleural streptokinase in children. Chest 125:566–570.
Wang, J. N., C. T. Yao, C. N. Yeh, C. C. Liu, M. H. Wu, H. Y. Chuang, and J. M. Wu. 2006. Once-daily versus twice-daily intrapleural urokinase treatment of complicated parapneumonic effusion in pediatric patients: a randomised, prospective study. Int. J. Clin. Pract. 60:1225–1230.
Huang, R. P., R. Huang, Y. Fan, and Y. Lin. 2001. Simultaneous detection of multiple cytokines from conditioned media and patient’s sera by an antibody-based protein array system. Anal. Biochem. 294:55– 62.
Light, R. W. 1995. A new classification of parapneumonic effusions and empyema. Chest 108:299–301.
Maskell, N. A., C. W. Davies, A. J. Nunn, E. L. Hedley, F. V. Gleeson, R. Miller, R. Gabe, G. L. Rees, T. E. Peto, M. A. Woodhead, D. J. Lane, J. H. Darbyshire, and R. J. Davies. 2005. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N. Engl. J. Med. 352:865–874.
Alemán, C., J. Alegre, J. Monasterio, R. M. Segura, L. Armadans, A. Anglés, E. Varela, E. Ruiz, and T. Fernández de Sevilla. 2003. Association between inflammatory mediators and the fibrinolysis system in infectious pleural effusions. Clin. Sci. 105:601–607.
Lin, F. C., Y. C. Chen, F. J. Chen, and S. C. Chang. 2005. Cytokines and fibrinolytic enzymes in tuberculous and parapneumonic effusions. Clin. Immunol. 116:166–173.
Chiu, C. Y., K. S. Wong, J. L. Huang, M. H. Tasi, T. Y. Lin, and S. Y. Hsieh. 2008. Proinflammatory cytokines, fibrinolytic system enzymes, and biochemical indices in children with infectious parapneumonic effusions. Pediatr. Infect. Dis. J. 27:699–703.
Iglesias, D., J. Alegre, C. Alemán, E. Ruíz, T. Soriano, L. I. Armadans, R. M. Segura, A. Anglés, J. Monasterio, and T. F. de Sevilla. 2005. Metalloproteinases and tissue inhibitors of metalloproteinases in exudative pleural effusions. Eur. Respir. J. 25:104–109.
Hurewitz, A. N., S. Zucker, P. Mancuso, C. L. Wu, B. Dimassimo, R. M. Lysik, and D. Moutsiakis. 1992. Human pleural effusions are rich in matrix metalloproteinases. Chest 102:1808–1814.
Rothenberg, M. E., N. Zimmermann, A. Mishra, E. Brandt, L. A. Birkenberger, S. P. Hogan, and P. S. Foster. 1999. Chemokines and chemokines receptors: their role in allergic airway disease. J. Clin. Immunol. 19:250–265.
Kalomenidis, I., K. H. Mohamed, K. B. Lane, R. S. Peebles, R. Barnette, R. M. Rodriguez, and R. W. Light. 2003. Pleural fluid levels of vascular cell adhesion molecule-1 are elevated in eosinophilic pleural effusions. Chest 124:159–166.
Acknowledgements
The authors thank Ms. Li-Wen Chen for the laboratory assistance; and Dr. Robert Anderson for editing the English. We would also like to acknowledge the contributions of Drs. Chih-Ta Yao, and Ching-Chuan Liu for patient management and sample collection.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Financial Support
This study was supported by the Intramural Research Program of the National Cheng Kung University Hospital (NCKUH-9803036), and Taiwan National Science Council (NSC 98-2314-B-006-007-MY3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, JN., Shin, JW., Chang, TY. et al. Decreased Proinflammatory Cytokines Production in Children with Complicated Parapneumonic Pleural Effusion after Intrapleural Fibrinolytic Treatment. Inflammation 32, 410–418 (2009). https://doi.org/10.1007/s10753-009-9150-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-009-9150-2