Abstract
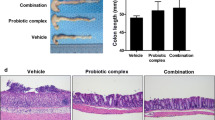
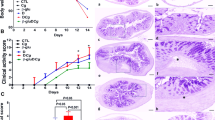
The biosynthesis and modification of mucopolysaccharides and glycosaminoglycans (GAGs), secreted from gastrointestinal mucosal cells, are increased in colitis and influence the viability of the defense barrier. Therefore, to evaluate the role of GAG-degrading intestinal microflora during the progression of colitis, we investigated the degradation activity of intestinal bacterial GAG, cytotoxicity of GAGs and their metabolites, such as iduronic acid, d-uronic acid or d-glucuronic acid and d-galactosamine or d-glucosamine, against intestinal cells. We also tested their deteriorative effects against colitis. Colitis was induced using 2,4,6-trinitrobenzene sulfonic acid (TNBS) with and without antibiotics in mice. The TNBS treatment caused colon shortening, increased myeloperoxidase activity, induced IL-1β, TNF-α, and IL-6 expression in the colon, activated NF-κB, and potentiated the GAG-degrading activities of intestinal microflora. The antibiotic treatment inhibited colon shortening, decreased myeloperoxidase activity, and reduced proinflammatory cytokine expression, NF-κB activation, and GAG degradation, induced by TNBS. Among the GAG metabolites, d-glucosamine and d-galactosamine showed cytotoxicity against intestinal cells, Caco-2 and IEC-18 cells, synergistically deteriorated the cytotoxicity of TNBS as well as the TNBS-induced colitis in mice. Based on these findings, intestinal microflora may degrade GAGs in colitis, their metabolites deteriorate the progress of colitis and antibiotics ameliorate the colitis by the inhibition of GAG-degrading bacterial growth.






Similar content being viewed by others
References
Benno, P., C. E. Leijonmarck, U. Monsen, and A. Uribe. 1993. Functional alterations of the microflora in patients with ulcerative colitis. Scand. J. Gastroenterol. 28:839–844. doi:10.3109/00365529309104019.
Gorbach, S. L., L. Nahas, A. G. Plaut, L. Weinstein, J. F. Patterson, and R. Levitan. 1968. Studies of intestinal microflora. V. Fecal microbial ecology in ulcerative colitis and regional enteritis: relationship to severity of disease and chemotherapy. Gastroenterology 54:575–587.
Chung, K. T., G. E. Fulk, and M. W. Slein. 1975. Tryptophanase of fecal flora as a possible factor in the etiology of colon cancer. J. Natl. Cancer Inst. 54:1073–1078.
Radema, S. A., S. J. van Deventer, and A. Cerami. 1991. Interleukin 1 beta is expressed predominantly by enterocytes in experimental colitis. Gastroenterology 100:1180–1186.
Rafii, F., R. van Embdin, and L. M. C. van Lieshout. 1999. Changes in bacterial enzymes and PCR profiles of fecal bacteria from a patient with ulcerative colitis before and after antimicrobial treatments. Dig. Dis. Sci. 44:637–642. doi:10.1023/A:1026634229934.
Berrebi, D., J. Languepin, L. Ferkdadji, A. Foussat, P. De Lagausie, R. Paris, D. Emilie, J. F. Mougenot, J. P. Cezard, J. Navarro, and M. Peuchmaur. 2004. Cytokines, chemokine receptors, and homing molecule distribution in the rectum and stomach of pediatric patients with ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 37:300–308. doi:10.1097/00005176-200309000-00018.
Binder, V. 2004. Epidemiology of IBD during the twentieth century: an integrated view. Best Pract. Res. Clin. Gastroenterol. 18:463–479. doi:10.1016/j.bpg.2003.12.002.
Chandran, P., S. Satthaporn, A. Robins, and O. Eremin. 2003. Inflammatory bowel disease: dysfunction of GALT and gut bacterial flora (II). Surgeon 1:125–136.
Rath, H. C., M. Schultz, R. Freitag, L. A. Dieleman, F. Li, H. J. Linde, J. Scholmerich, and R. B. Sartor. 2001. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect. Immun. 69:2277–2285. doi:10.1128/IAI.69.4.2277-2285.2001.
Neurath, M., I. Fuss, and W. Strober. 2000. TNBS-colitis. Int. Rev. Immunol. 19:51–62. doi:10.3109/08830180009048389.
Hill, M. J., and B. S. Drasar. 1975. The normal colonic bacterial flora. Gut 16:318–323. doi:10.1136/gut.16.4.318.
Ganguly, N. K. , J. G. Kingham, B. Lloyd, R. S. Lloyd, C. P. Price, D. R. Triger, and R. Wright. 1978. Acid hydrolases in monocytes from patients with inflammatory bowel disease, chronic liver disease, and rheumatoid arthritis. Lancet 1(8073):1073–1075.
Rhodes, J. M., R. Gallimore, and E. Elias. 1985. Faecal mucus degrading glycosidases in ulcerative colitis and Crohn's disease. Gut 26:761–765. doi:10.1136/gut.26.8.761.
Rifkin, G. D., J. Silva Jr., and R. Fekety. 1978. Gastrointestinal and systemic toxicity of fecal extracts from hamsters with clindamycin-induced colitis. Gastroenterology 74:52–57.
Wallace, J. L., W. K. Macnaughton, G. P. Morris, and P. L. Beck. 1989. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology 96:29–36.
Belmiro, C. L., H. S. Souza, C. C. Elia, M. T. Castelo-Branco, F. R. Silva, R. L. Machado, and M. S. Pavao. 2005. Biochemical and immunohistochemical analysis of glycosaminoglycans in inflamed and non-inflamed intestinal mucosa of patients with Crohn's disease. Int. J. Colorectal. Dis. 20:295–304. doi:10.1007/s00384-004-0677-2.
Gesner, B. M., and C. R. Jenkin. 1961. Production of heaprinase by bacteroides. J. Bacteriol. 81:595–604.
Corfield, A. P., N. Myerscough, N. Bradfield, C. D. A. Corfield, M. Gough, J. R. Clamp, P. Durdey, B. F. Warren, D. C. C. Bartolo, K. R. King, and J. M. Williams. 1996. Colonic mucins in ulcerative colitis: evidence for loss of sulfation. Glycoconjugate J. 13:89–822. doi:10.1007/BF00702345.
Greenberg, G. R. 2004. Antibiotics should be used as first-line therapy for Crohn’s disease. Inflamm. Bowel Dis. 10:318–320. doi:10.1097/00054725-200405000-00021.
Sartir, R. B. 2004. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 126:1620–1633. doi:10.1053/j.gastro.2004.03.024.
Mullane, K. M., R. Kraemer, and B. Smith. 1985. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J. Pharmacol. Methods 14:157–167. doi:10.1016/0160-5402(85)90029-4.
Choo, M. K., N. Kawasaki, P. Singhirunnusorn, K. Koizumi, S. Sato, S. Akira, I. Saiki, and H. Sakurai. 2006. Blockade of transforming growth factor-beta-activated kinase 1 activity enhances TRAIL-induced apoptosis through activation of a caspase cascade. Mol. Cancer Ther. 5:2970–2976. doi:10.1158/1535-7163.MCT-06-0379.
Lee, D. S., Y. S. Kim, C. N. Ko, K. H. Cho, H. S. Bae, K. S. Lee, J. J. Kim, E. K. Park, and D. H. Kim. 2002. Fecal metabolic activities of herbal components to bioactive compounds. Arch. Pharm. Res. 25:165–169. doi:10.1007/BF02976558.
Carmichael, J., W. G. DeGreff, A. F. Gazdar, J. D. Minna, and J. B. Mitchell. 1987. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 47:936–940.
Keighley, M. R., and Y. Arabi. 1978. Influence of inflammatory bowel disease on intestinal microflora. Gut 19:1099–1104. doi:10.1136/gut.19.12.1099.
Onderdonk, A. B., J. A. Aermos, and J. G. Barblett. 1979. The role of the intestinal microflora in experimental colitis. Am. J. Clin. Nutr. 30:1819–1825.
Appleton, I., A. Tomlinsom, and D. A. Willoughby. 1996. Induction of cyclo-oxygenase and nitric oxide synthase in inflammation. Adv. Pharmacol. 35:27–78. doi:10.1016/S1054-3589(08)60274-4.
Sartor, R. B. 1994. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology 106:533–539.
Singer, I. I., D. W. Kawka, S. Schloemann, R. Tessner, T. Riehl, and W. F. Stenson. 1998. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 115:297–306. doi:10.1016/S0016-5085(98)70196-9.
Gesner, B. M., and C. R. Jenkin. 1961. Production of heparinase by Bacteroides. J. Bacteriol. 81:595–604.
Salyers, A. A., J. R. Vercellotti, S. H. E. West, and T. D. Wilkins. 1977. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl. Environ. Microbiol. 34:319–322.
Hong, S. W., B. T. Kim, H. Y. Shin, W. S. Kim, K. S. Lee, Y. S. Kim, and D. H. Kim. 2002. Purification and characterization of novel chondroitin ABC and AC lyases from Bacteroides stercoris HJ-15, a human intestinal anaerobic bacterium. Eur. J. Biochem. 269:2934–2940.
Salyers, A. A., M. Pajeau, and R. E. McCarthy. 1988. Importance of mucopolysaccharides as substrates for Bacteroides thetaiotaomicron growing in intestinal tracts of exgermfree mice. Appl. Environ. Microbiol. 54:1970–1976.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, HS., Han, SY., Ryu, KY. et al. The Degradation of Glycosaminoglycans by Intestinal Microflora Deteriorates Colitis in Mice. Inflammation 32, 27–36 (2009). https://doi.org/10.1007/s10753-008-9099-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-008-9099-6