Abstract
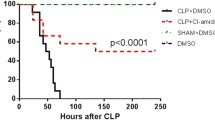
Zymosan-induced generalized inflammation is a convenient model to study the process of acute and chronic inflammatory processes resulting in multiple organ dysfunction syndrome. Macrophages as a source of many pro-inflammatory mediators are the major players in shock and further organ failure. Etoposide is a cytostatic drug known to reduce macrophages and monocytes in blood circulation. In the present study we have investigated whether the ability of etoposide to diminish macrophage number would have an impact on the course of zymosan-induced shock. The drug injected at a dose of 10 mg/kg 1 day before zymosan, significantly reduced the mortality and decreased the organ toxicity in Balb/c mice. Simultaneously, an inhibition of TNF-α production by alveolar and peritoneal macrophages was observed. Etoposide administered into mice with severe combined immunodeficiency (SCID) did not change the survival rate and had a little influence on organ toxicity. Our findings suggest that the beneficial action of etoposide might be attributed to the reduction of macrophages and alteration of their functions. Its effect depends on the presence of functional T and B lymphocytes. The results deserve further investigation of etoposide as a perspective therapeutic tool for inhibiting the excessive inflammatory response and to be helpful for revealing mechanisms of shock development.





Similar content being viewed by others
References
Deitch, E. A. 1998. Animal models of sepsis and shock: a review and lessons learned. Shock 9:1–11.
Carpati, C. M., M. E. Astiz, and E. C. Rackow. 1999. Mechanisms and management of myocardial dysfunction in septic shock. Crit. Care. Med. 27:231–232.
Karima, R., S. Matsumoto, H. Higashi, and K. Matsushima. 1999. The molecular pathogenesis of endotoxic shock and organ failure. Mol. Med. Today 5:123–132.
Davies, M. G., and P. O. Hagen. 1997. Systemic inflammatory response syndrome. Br. J. Surg. 84:920–935.
Nelson, S. 1999. A question of balance. Am. J. Respir. Crit. Care Med. 159:1365–1367.
Goris, R. J., W. K. Boekholtz, I. P. van Bebber, J. K. Nuytinck, and P. H. Schillings. 1986. Multiple-organ failure and sepsis without bacteria. An experimental model. Arch. Surg. 121:897–901.
Underhill, D. M. 2003. Macrophage recognition of zymosan particles. J. Endotoxin. Res. 9:176–180.
Meng, G., A. Grabiec, M. Rutz, J. Metzger, P. B. Luppa, H. Wagner, S. Bauer, and C. J. Kirschning. 2005. Murine TLR2 expression analysis and systemic antagonism by usage of specific monoclonal antibodies. Immunol. Lett. 98:200–207.
Sfeir, T., D. C. Saha, M. Astiz, and E. C. Rackow. 2001. Role of interleukin-10 in monocyte hyporesponsiveness associated with septic shock. Crit. Care Med. 29:129–133.
von Asmuth, E. J., J. G. Maessen, C. J. van der Linden, and W. A. Buurman. 1990. Tumour necrosis factor alpha (TNF-alpha) and interleukin 6 in a zymosan-induced shock model. Scand. J. Immunol. 32:313–319.
Aldridge, A. J. 2002. Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur. J. Surg. 168:204–214.
Baker, C. C., and M. D. Huynh. 1995. Sepsis in the critically ill patient. Curr. Probl. Surg. 32:1013–1083.
Aisner, J., and E. J. Lee. 1991. Etoposide. Current and future status. Cancer 67:215–219.
Hande, K. R. 1998. Etoposide: four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 34:1514–1521.
Johnson, D. H., J. D. Hainsworth, K. R. Hande, and F. A. Greco. 1991. Current status of etoposide in the management of small cell lung cancer. Cancer 67:231–244.
Beutler, B., and A. Cerami. 1989. The biology of cachectin/TNF-a primary mediator of the host response. Annu. Rev. Immunol. 7:625–655.
Blackwell, T. S., and J. W. Christman. 1996. Sepsis and cytokines: current status. Br. J. Anaesth. 77:110–117.
Burdon, D., T. Tiedje, K. Pfeffer, E. Vollmer, and P. Zabel. 2000. The role of tumor necrosis factor in the development of multiple organ failure in a murine model. Crit. Care Med. 28:1962–1967.
Calame, W., A. E. Douwes-Idema, M. T. van den Barselaar, R. van Furth, and H. Mattie. 1994. Influence of cytostatic agents on the pulmonary defence of mice infected with Klebsiella pneumoniae and on the efficacy of treatment with ceftriaxone. J. Infect. 29:53–66.
Calame, W., R. van der Waals, H. Mattie, and R. van Furth. 1989. Influence of etoposide and cyclophosphamide on the efficacy of cloxacillin and erythromycin in an experimental staphylococcal infection. Antimicrob. Agents Chemother. 33:980–982.
Verdrengh, M., O. Isaksson, and A. Tarkowski. 2005. Topoisomerase II inhibitors, irrespective of their chemical composition, ameliorate experimental arthritis. Rheumatology (Oxford) 44:183–186.
Verdrengh, M., and A. Tarkowski. 2003. Impact of topoisomerase II inhibition on cytokine and chemokine production. Inflamm. Res. 52:148–153.
Nieuwenhuijzen, G. A., M. F. Knapen, T. Hendriks, N. van Rooijen, and R. J. Goris. 1997. Elimination of various subpopulations of macrophages and the development of multiple-organ dysfunction syndrome in mice. Arch. Surg. 132:533–539.
Jansen, M. J., T. Hendriks, M. T. Vogels, J. W. van der Meer, and R. J. Goris. 1996. Inflammatory cytokines in an experimental model for the multiple organ dysfunction syndrome. Crit. Care Med. 24:1196–1202.
Volman, T. J., R. J. Goris, M. van der Jagt, F. A. van de Loo, and T. Hendriks. 2002. Organ damage in zymosan-induced multiple organ dysfunction syndrome in mice is not mediated by inducible nitric oxide synthase. Crit. Care Med. 30:1553–1559.
Seiter, K. 2005. Toxicity of the topoisomerase II inhibitors. Expert. Opin. Drug Saf. 4:219–234.
Goldmann, O., G. S. Chhatwal, E. Medina. 2005. Contribution of natural killer cells to the pathogenesis of septic shock induced by Streptococcus pyogenes in mice. J. Infect. Dis. 191:1280–1286.
Acknowledgement
This work was supported by the fellowship grant MU 501/02.09.2005 to National Science Fund, Ministry of Education and Science, Bulgaria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Remichkova, M., Yordanov, M. & Dimitrova, P. Etoposide Attenuates Zymosan-Induced Shock in Mice. Inflammation 31, 57–64 (2008). https://doi.org/10.1007/s10753-007-9049-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-007-9049-8