Abstract
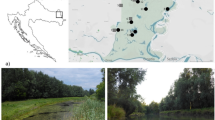
This study evaluated periphyton biomass, nutrient content, and taxonomical composition in three nutrient-poor post-mining lakes in the Czech Republic. Two methods, microscopy and chemotaxonomy, were used to determine the taxonomical composition of autotrophs. Both methods identified diatoms, Chlorophyta, and Cyanobacteria as the dominant groups across the lakes. Considerable congruence of the taxonomical methods was found for diatoms and Chlorophyta, however results for Cyanobacteria showed poor correlation. The differences in periphyton features among the lakes were mostly explained by the lake age and trophy. Moreover, high amounts of overwintering biomass show that periphyton development is not established “de novo” each year but its current stage is a cumulative result of previous years. Beside the lake age and trophy, limnological characteristics such as Si or Mg2+ also affect periphyton taxonomical composition. No correlation of periphytic C:N:P molar ratios with lake water nitrogen and phosphorus, suggests role of additional process to the nutrient uptake, likely internal nutrient recycling in periphyton. These findings are essential in predicting further succession in the examined post-mining lakes and serve as a model for newly formed lakes. As more lakes will be formed within the post-mining recultivation in the short horizon, our study contributes to their successful management.





Similar content being viewed by others
Data availability
Data are available from the authors upon reasonable request.
References
APHA, 1985. Standard Methods for the Examination of Water and Waste-Water, 16th ed. American Public Health Association, Washington, DC:
APHA, 2017. Standard Methods for the Examination of Water and Wastewater, 23rd ed. American Public Health Association, Washington, DC:
Asaeda, T., V. K. Trung & J. Manatunge, 2000. Modeling the effects of macrophyte growth and decomposition on the nutrient budget in Shallow Lakes. Aquatic Botany 68: 217–237. https://doi.org/10.1016/S0304-3770(00)00123-6.
Atkins, K. S., S. H. Hackley, B. C. Allen, S. Watanabe, J. E. Reuter & S. G. Schladow, 2021. Variability in periphyton community and biomass over 37 years in Lake Tahoe (CA-NV). Hydrobiologia 848(8): 1755–1772. https://doi.org/10.1007/s10750-021-04533-w.
Axler, R., S. Yokom, C. Tikkanen, M. McDonald, H. Runke, D. Wilcox & B. Cady, 1998. Restoration of a mine pit lake from aquacultural nutrient enrichment. Restoration Ecology 6(1): 1–19. https://doi.org/10.1046/j.1526-100x.1998.00612.x.
Azim, M. E., M. C. J. Verdegem, A. A. van Dam & M. C. M. Beveridge, 2005. Periphyton: Ecology, Exploitation and Management, CABI, Wallingford:
Bešta, T., J. Mareš, K. Čapková, E. Janeček, L. Štenclová, A. Kust, M. Říha, E. Konopáčová & Klára. Řeháková, 2023. Littoral periphyton dynamics in newly established post-mining lakes. Aquat Sci 85: 21. https://doi.org/10.1007/s00027-022-00914-y.
Braun-Blanquet, J., 1921. Prinzipien einer Systematik der Pflanzengesellschaften auf floristischer Grundlage. Jahrb. St. Gallen Naturwiss. Ges. 57-II: 305–351.
Buchaca, T., S. Kosten, G. Lacerot, N. Mazzeo, C. Kruk, V. Huszar, L. Huszar, A. Lotter & E. Jeppesen, 2019. Pigments in surface sediments of South American shallow lakes as an integrative proxy for primary producers and their drivers. Freshwater Biology 64: 1–16. https://doi.org/10.1111/fwb.13317.
Cantonati, M. & R. L. Lowe, 2014. Lake benthic algae: toward an understanding of their ecology. Freshwater Science 33(2): 475–486. https://doi.org/10.1086/676140.
Carlson, R. E., 1977. A trophic state index for lakes. Limnology and Oceanography 22: 361–369.
Carlson, R. E. & J. Simpson, 1996. A coordinator’s guide to volunteer lake monitoring methods. North American Lake Management Society 96: 305.
CSN EN ISO 10260:1992 Jakost vod. Měření biochemických ukazatelů. Spektrofotometrické stanovení koncentrace chlorofylu-a, Czech Standard.
CSN EN ISO 11885:1996. Jakost vod – Stanovení 33 prvků atomovou emisní spektrometrií s indukčně vázaným plazmatem (ICP AES), Czech Standard.
CSN EN ISO 9963:1996. Jakost vod. Stanovení kyselinové neutralizační kapacity (KNK). Část 1: Stanovení KNK4,5 a KNK8,3, Czech Standard.
Dalton, R. L., C. Boutin & F. R. Pick, 2015. Determining in situ periphyton community responses to nutrient and atrazine gradients via pigment analysis. Science of the Total Environment 515–516: 70–82. https://doi.org/10.1016/j.scitotenv.2015.01.023.
DeNicola, D. M. & M. G. Kelly, 2014. Role of periphyton in ecological assessment of lakes. Freshwater Science. https://doi.org/10.1086/676117.
Dudley, J. L., W. Arthurs & T. J. Hall, 2001. A comparison of methods used to estimate river rock surface areas. Journal of Freshwater Ecology 16(2): 257–261. https://doi.org/10.1080/02705060.2001.9663810.
Esposito, E. & S. F. Abramson, 2021. The European coal curse. Journal of Economic Growth 26: 77–112. https://doi.org/10.1007/s10887-021-09187-w.
Ettl, H. & Gaertner, G., 1988. Chlorophyta II – Tetrasporales, Chlorococcales, Gloeodendrales. Süßwasserflora von Mitteleuropa. Band 10. Gustav Fischer Verlag, Springer, Jena.
European Commission, 2000. Water Framework Directive.
European Commission, 2018. A Clean Planet for All.
Ferrarezi, R. S., X. Lin, A. C. Gonzalez Neira, F. Tabay Zambon, H. Hu, X. Wang, J.-H. Huang & G. Fan, 2022. Substrate pH influences the nutrient absorption and rhizosphere microbiome of Huanglongbing-affected grapefruit plants. Frontiers in Plant Science 13: 856937. https://doi.org/10.3389/fpls.2022.856937.
Fong, P., T. C. Foin & J. B. Zedler, 1994. A simulation model of lagoon algae based on nitrogen competition and internal storage. Ecological Monographs 64(2): 225–247. https://doi.org/10.2307/2937042.
Frouz, J., 2021. Soil recovery and reclamation of mined lands. In Soils and Landscape Restoration. 161–91. https://doi.org/10.1016/B978-0-12-813193-0.00006-0.
Gaiser, E., 2009. Periphyton as an indicator of restoration in the Florida everglades. Ecological Indicators 9(6): 37–45. https://doi.org/10.1016/j.ecolind.2008.08.004.
Gammons, Ch. H., L. Harris, J. Castro, P. Cott & B. Hanna, 2009. Creating lakes from open pit mines: processes and considerations, with emphasis on northern environments. Canadian Technical Report of Fisheries and Aquatic Sciences.
Gammons, Ch. H., B. L. Pape, S. R. Parker, S. R. Poulson & C. E. Blank, 2013. Geochemistry, water balance, and stable isotopes of a “clean” pit lake at an abandoned tungsten mine, Montana, USA. Applied Geochemistry 36: 57–69. https://doi.org/10.1016/j.apgeochem.2013.06.011.
Havens, K. E., A. D. Steinman, H. J. Carrick, J. W. Louda, N. M. Winfree & E. W. Baker, 1999. Comparative analysis of lake periphyton communities using high performance liquid chromatography (HPLC) and light microscope counts. Aquatic Sciences 61(4): 307–322. https://doi.org/10.1007/s000270050068.
Higgins, H. W., S. W. Wright & L. Schlüter, 2011. Quantitative interpretation of chemotaxonomic pigment data. In Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography. 257–313. https://doi.org/10.1017/CBO9780511732263.010.
Hill, B. H., A. T. Herlihy, P. R. Kaufmann, R. J. Stevenson, F. H. McCormick & C. B. Johnson, 2000. Use of periphyton assemblage data as an index of biotic integrity. Journal of the North American Benthological Society 19(1): 50–67. https://doi.org/10.2307/1468281.
Hillebrand, H. & U. Sommer, 1999. The nutrient stoichiometry of benthic microalgal growth: redfield proportions are optimal. Limnology and Oceanography. https://doi.org/10.4319/lo.1999.44.2.0440.
Hindák, F., 1978. Sladkovodné Riasy, SPN, Bratislava:
Hindák, F., 1996. Klúč Na Určovanie Nerozkonárených Vláknitých Zelených Rias (Ulotrichineae, Ulotrichales, Chlorophyceae). [Key for Determination of Filamentous Green Algae (Ulotrichineae, Ulotrichales, Chlorophyceae)], Bulletin Slovenskej Botanickej Spoločnosti Pri SAV, Bratislava:
Hooke, R., J. M. Duque & J. de Pedraza, 2012. Land transformation by humans: a review. GSA Today 22: 4–10. https://doi.org/10.1130/GSAT151A.1.
Iannino, A., A. T. L. Vosshage, M. Weitere & P. Fink, 2020. Taxonomic shift over a phosphorus gradient affects the stoichiometry and fatty acid composition of stream periphyton. Journal of Phycology 56(6): 1687–1695. https://doi.org/10.1111/jpy.13060.
Jäger, Ch. G. & S. Diehl, 2014. Resource competition across habitat boundaries: asymmetric interactions between benthic and pelagic producers. Ecological Monographs 84(2): 287–302. https://doi.org/10.1890/13-0613.1.
Jerney, J., M. Mayr & M. Schagerl, 2016. Biofilm scrubbing for restoration – algae community composition and succession in artificial streams. AIMS Environmental Science 3: 560–581. https://doi.org/10.3934/environsci.2016.3.560.
Kahlert, M., 1998. C:N:P ratios of freshwater benthic algae. Archiv für Hydrobiologie (Advances in Limnology) 51: 105–14.
Kaštovský, J., T. Hauer, R. Geriš, B. Chattová, J. Juráň, O. Skácelová, P. Pitelková, M. Pusztai, P. Škaloud, J. Šťastný, K. Čapková, M. Bohunická & R. Muhlsteinova, 2018a. Atlas Sinic a Řas ČR 1. [Atlas of cyanobateria and algae of the Czech Republic 1], University of South Bohemia in České Budějovice, Praha:
Kaštovský, J., T. Hauer, R. Geriš, B. Chattová, J. Juráň, O. Skácelová, P. Pitelková, M. Pusztai, P. Škaloud, J. Šťastný, K. Čapková, M. Bohunická & R. Muhlsteinova, 2018b. Atlas Sinic a Řas ČR 2. [Atlas of cyanobateria and algae of the Czech Republic 2], University of South Bohemia in České Budějovice, Praha:
Keshri, J., P. R. Angia Sriram, J. Colombet, F. Perriere, T. Antoine & T. Sime-Ngando, 2017. Differential impact of lytic viruses on the taxonomical resolution of freshwater bacterioplankton community structure. Water Research 124: 129–138. https://doi.org/10.1016/j.watres.2017.07.053.
Kitner, M. & A. Poulíčková, 2003. Littoral diatoms as indicators for the eutrophication of shallow lakes. Hydrobiologia 506–509: 519–524. https://doi.org/10.1023/B:HYDR.0000008567.99066.92.
Komárek, J. & K. Anagnostidis, 1998. Cyanoprokaryota 1. Teil: Chroococcales. In Ettl, H., G. Gärtner, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa 19/1. Gustav Fischer, Jena-Stuttgart-Lübeck-Ulm.
Komárek, J. & K. Anagnostidis, 2005. Cyanoprokaryota -2. Teil/ 2nd Part: Oscillatoriales. In Büdel, B., L. Krienitz, G. Gärtner & M. Schagerl (eds), Süsswasserflora von Mitteleuropa 19/2. Elsevier/Spektrum, Heidelberg.
Komárek, J. & B. Fott, 1983. Chlorococcales. In Huber-Pestalozzi, G. (ed), Das Phytoplankton Des Süßwassers. Die Binnengewässer 16, 7/1. Schweizerbartsche Verlagsbuchhandlung, Stuttgart.
Konopáčová, E., J. Nedoma, K. Čapková, P. Čapek, P. Znachor, M. Pouzar, M. Říha & K. Řeháková, 2021. Low specific phosphorus uptake affinity of epilithon in three oligo- to mesotrophic post-mining lakes. Frontiers in Microbiology 12: 735498. https://doi.org/10.3389/fmicb.2021.735498.
Kopáček, J. & J. Hejzlar, 1993. Semi-micro determination of total phosphorus in freshwaters with perchloric acid digestion. International Journal of Environmental Analytical Chemistry 53: 173–183. https://doi.org/10.1080/03067319308045987.
Krammer, K., 2000. Diatoms of Europe. Diatoms of the European Inland Waters and Comparable Habitats. The Genus Pinnularia. Vol 1. A.R.G. Gantner Verlag K.G., Ruggell: 703.
Krammer, K.., 2002. Diatoms of Europe. Diatoms of the European Inland Waters and Comparable Habitats. Cymbella. Vol. 3. A.R.G. Gantner Verlag K.G., Ruggell: 584.
Krammer, K., 2003. Diatoms of Europe. Diatoms of the European Inland Waters and Comparable Habitats. Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella. Vol. 4. A.R.G. Gantner Verlag K.G., Ruggell: 529.
Krammer, K. & H. Lange-Bertalot, 1986. Bacillariophyceae. 1. teil: naviculaceae. In Ettl, H., J. Gerloff, H. Heynig, D. Mollenhaurer (eds), Süsswasserflora von Mitteleuropa. Band 2/1. Gustav Fisher Verlag, Jena: 876.
Krammer, K. & H. Lange-Bertalot, 1988. Bacillariophyceae. 2. teil: bacillariaceae, epithemiaceae, surirellaceae. In Ettl, H., J. Gerloff, H. Heynig, D. Mollenhaurer (eds), Susswasserflora von Mitteleuropa. Band 2/2. Gustav Fisher Verlag, Jena: 610.
Krammer, K. & H. Lange-Bertalot, 1991a. Bacillariophyceae. 3. teil: centrales, fragilariaceae, eunotiaceae. In Ettl, H., J. Gerloff, H. Heynig, D. Mollenhaurer (eds), Süsswasserflora von Mitteleuropa. Band 2/3. Gustav Fisher Verlag, Jena: 598.
Krammer, K. & H. Lange-Bertalot, 1991b. Bacillariophyceae. 4. teil: achnanthaceae, kritische ergänzungen zu navicula (lineolatae) und gomphonema gesamtliteraturverzeichnis. In Ettl, H., J. Gerloff, H. Heynig, D. Mollenhaurer (eds), Süsswasserflora von Mitteleuropa.
Lambert, D., A. Cattaneo & R. Carignan, 2008. Periphyton as an early indicator of perturbation in recreational lakes. Canadian Journal of Fisheries and Aquatic Sciences. 65(2): 258–265. https://doi.org/10.1139/f07-168.
Lange-Bertalot, H., 2001. Diatoms of Europe. Diatoms of European Inland Waters and Comparable Habitats. Navicula sensu stricto, 10 Genera Separated from Navicula sensu lato, Frustulia. Vol. 2. A.R.G. Gantner Verlag K.G., Ruggell: 526.
Larondelle, N. & D. Haase, 2012. Valuing post-mining landscapes using an ecosystem services approach – an example from Germany. Ecological Indicators 18: 567–574. https://doi.org/10.1016/j.ecolind.2012.01.008.
Lauridsen, T., L. Schlüter & L. Johansson, 2011. Determining algal assemblages in oligotrophic lakes and streams: comparing information from newly developed pigment/chlorophyll a ratios with direct microscopy. Freshwater Biology 56: 1638–1651. https://doi.org/10.1111/j.1365-2427.2011.02588.x.
Liess, A., S. Drakare & M. Kahlert, 2009. Atmospheric nitrogen-deposition may intensify phosphorus limitation of shallow epilithic periphyton in unproductive lakes. Freshwater Biology 54: 1759–1773. https://doi.org/10.1111/j.1365-2427.2009.02222.x.
Lindstrom, E. S., M. P. Kamst-Van Agterveld & G. Zwart, 2005. Distribution of typical freshwater bacterial groups is associated with pH, temperature, and lake water retention time. Applied and Environmental Microbiology 71: 8201–8206. https://doi.org/10.1128/AEM.71.12.8201-8206.2005.
Llirós, M., Ö. Inceoğlu, T. García-Armisen, A. Anzil, B. Leporcq, L. M. Pigneur, L. Viroux, F. Darchambeau, J. P. Descy & P. Servais, 2014. Bacterial community composition in three freshwater reservoirs of different alkalinity and trophic status. PLoS ONE 9: e116145. https://doi.org/10.1371/journal.pone.0116145.
Louda, W., C. Grant, J. Browne & S. Hagerthey, 2015. Pigment-based chemotaxonomy and its application to everglades periphyton. In Microbiology of the Everglades Ecosystem. 289–349. https://doi.org/10.1201/b18253-16.
Lowe, R. L. & Y. Pan, 1996. Benthic algal communities and biological monitors. 705739. In: Stevenson, R. J., Bothwell, M. L., and Lowe, R. L. (eds), Algal Ecology: Freshwater Benthic Ecosystems. New York, New York: Academic Press. 753 p.
Luhtala, H. & H. Tolvanen, 2013. Optimizing the use of Secchi depth as a proxy for euphotic depth in coastal waters: an empirical study from the Baltic Sea. ISPRS International Journal of Geo-Information 2: 1153–1168. https://doi.org/10.3390/ijgi2041153.
Mackereth, F. J. H., J. Talling & J. F. Heron, 1978. Water Analysis: Some Revised Methods for Limnologists, Freshwater Biological Association, Cumbria:
Mackey, M. D., D. J. Mackey, H. W. Higgins & S. Wright, 1996. CHEMTAX – a program for estimating class abundances from chemical markers: application to HPLC measurements of phytoplankton. Marine Ecology - Progress Series 144: 265–283. https://doi.org/10.3354/meps144265.
Mackey, M. D., D. J. Mackey, H. W. Higgins & S. Wright, 1997. CHEMTAX – a program for estimating class abundances from chemical markers: application to HPLC measurements of phytoplankton. Oceanographic Literature Review 7(44): 716. https://doi.org/10.4225/08/585eb90c906fe.
Marazzi, L. & E. E. Gaiser, 2018. Long-term changes in spatially structured benthic diatom assemblages in a major subtropical wetland under restoration. Inland Waters 8(4): 434–448. https://doi.org/10.1080/20442041.2018.1500206.
Martin-Jezequel, V., M. Hildebrand & M. Brzezinski, 2000. Silicon metabolism in diatoms: implications for growth. Journal of Phycology 36: 821–840. https://doi.org/10.1046/j.1529-8817.2000.00019.x.
Michelutti, N., M. S. V. Douglas, D. R. S. Lean & J. P. Smol, 2002. Physical and chemical limnology of 34 ultra-oligotrophic lakes and ponds near WynniattBay, Victoria Island, Arctic Canada. Hydrobiologia 482: 1–13. https://doi.org/10.1023/A:1021201704844.
Mooney, T. J., C. D. McCullough, A. Jansen, L. Chandler, M. Douglas, A. J. Harford, R. van Dam & C. Humphrey, 2020. Elevated magnesium concentrations altered freshwater assemblage structures in a mesocosm experiment. Environmental Toxicology and Chemistry 39: 1973–1987. https://doi.org/10.1002/etc.4817.
Murphy, J. & J. P. Riley, 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27(1): 31–36. https://doi.org/10.1016/S0003-2670(00)88444-5.
Oberholster, P. J., Y. Schoeman, J. C. Truter & A.-M. Botha, 2022. Using periphyton assemblage and water quality variables to assess the ecological recovery of an ecologically engineered wetland affected by acid mine drainage after a dry spell. Processes 10(5): 877. https://doi.org/10.3390/pr10050877.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara & H. Wagner, 2019. Vegan: community ecology package. R package version 2.5-6. https://CRAN.R-project.org/package=vegan.
Pacheco, J. P., C. Calvo, C. Aznarez, M. Barrios, M. Meerhoff, E. Jeppesen & A. Baattrup-Pedersen, 2022. Periphyton biomass and life-form responses to a gradient of discharge in contrasting light and nutrients scenarios in experimental lowland streams. Science of The Total Environment. https://doi.org/10.1016/j.scitotenv.2021.150505.
Peng, X., K. Yi, O. Lin, L. Zhang, Y. Zhang, B. Liu & Z. Wu, 2020. Annual changes in periphyton communities and their diatom indicator species, in the littoral zone of a subtropical urban lake restored by submerged plants. Ecological Engineering 155: 105958. https://doi.org/10.1016/j.ecoleng.2020.105958.
Pieczyńska, E., 1993. Detritus and nutrient dynamics in the shore zone of lakes: a review. Hydrobiologia 251: 49–58. https://doi.org/10.1007/BF00007164.
Procházková, L., 1959. Bestimmung Der Nitrate Im Wasser. Fresenius’ Zeitschrift Für Analytische Chemie 167(4): 254–260. https://doi.org/10.1007/BF00458786.
R Core Team, 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
Riber, H. H. & R. G. Wetzel, 1987. Boundary-layer and internal diffusion effects on phosphorus fluxes in lake periphyton 1. Limnology and Oceanography 32: 1181–1194. https://doi.org/10.4319/lo.1987.32.6.118.
Rodríguez, P. & H. Pizarro, 2015. Phytoplankton and periphyton production and its relation to temperature in a humic lagoon. Limnologica 55: 9–12. https://doi.org/10.1016/j.limno.2015.10.003.
Schaumburg, J., Ch. Schranz, G. Hofmann, D. Stelzer, S. Schneider & U. Schmedtje, 2004. Macrophytes and phytobenthos as indicators of ecological status in German Lakes – a contribution to the implementation of the water framework directive. Limnologica 34(4): 302–314. https://doi.org/10.1016/S0075-9511(04)80003-3.
Schmidt, A. & M. Schubert, 2007. Using radon-222 for tracing groundwater discharge into an open-pit lignite mining lake – a case study. Isotopes in Environmental and Health Studies 43(4): 387–400. https://doi.org/10.1080/10256010701705419.
Schultze, M., K. H. Pokrandt & W. Hille, 2010. Pit lakes of the Central German lignite mining district: creation, morphometry and water quality aspects. Limnologica 40(2): 148–155. https://doi.org/10.1016/j.limno.2009.11.006.
Sickman, J., A. Albertin, M. W. Anderson, A. Pinowska & R. Stevenson, 2009. A comparison of internal and external supply of nutrients to algal mats in two first magnitude springs in Florida. Journal of Aquatic Plant Management 47: 135–144.
Simmons, L., C. Sandgren & J. Berges, 2016. Problems and pitfalls in using HPLC pigment analysis to distinguish Lake Michigan phytoplankton taxa. Journal of Great Lakes Research. https://doi.org/10.1016/j.jglr.2015.12.006.
Søndergaard, M., T. L. Lauridsen, L. S. Johansson & E. Jeppesen, 2018. Gravel pit lakes in Denmark: chemical and biological state. Science of the Total Environment 612: 9–17. https://doi.org/10.1016/j.scitotenv.2017.08.163.
Stevenson, R. J., Bothwell, M. L., and Lowe, R. L. (eds), Algal Ecology: Freshwater Benthic Ecosystems. Academic Press. Cambridge, 705–739. https://doi.org/10.1016/B978-0-12-668450-6.X5027-9
Vadeboncoeur, Y. & A. Steinman, 2002. Periphyton function in lake ecosystems. Scientific World Journal 2: 1449–1468. https://doi.org/10.1100/tsw.2002.294.
Vadeboncoeur, Y., M. V. Moore, S. D. Stewart, S. Chandra, K. S. Atkins, J. S. Baron, K. Bouma-Gregson, S. Brothers, S. N. Francoeur & L. Genzoli, 2021. Blue waters, green bottoms: benthic filamentous algal blooms are an emerging threat to clear lakes worldwide. BioScience 71: 1011–1027. https://doi.org/10.1093/biosci/biab049.
Van Dam, R. A., A. C. Hogan, C. D. McCullough, M. A. Houston, C. L. Humphrey & A. J. Harford, 2010. Aquatic toxicity of magnesium sulfate, and the influence of calcium, in very low ionic concentration water. Environmental Toxicology and Chemistry 29: 410–421. https://doi.org/10.1002/etc.56.
Van Heukelem, L. & C. S. Thomas, 2001. Computer-assisted high performance liquid chromatogaphy method development with applications to the isolation and analysis of phytoplankton pigments. Journal of Chromatography A 910: 31–49. https://doi.org/10.1016/S0378-4347(00)00603-4.
Vejříková, I., L. Vejřík, M. Čech, M. Říha & J. Peterka, 2022. Succession of submerged vegetation in a hydrologically reclaimed opencast mine during first 10 years. Restoration Ecology 30: e13489. https://doi.org/10.1111/rec.13489.
Vrablik, P., E. Wildova & J. Vrablikova, 2017. The effect of brown coal mining on the environment and health of the population in Northern Bohemia (Czech Republic). International Journal of Clean Coal and Energy 06(01): 1–13. https://doi.org/10.4236/ijcce.2017.61001.
Weber, C. I., 1973. Biological field and laboratory methods for measuring the quality of surface waters and effluents. United States E.P.A. Report 670/4/73/001.
Xiang, J., V. R. Haden, S. Peng, B. A. M. Bouman, R. M. Visperas, L. Nie, J. Huang & K. Cui, 2009. Improvement in nitrogen availability, nitrogen uptake and growth of aerobic rice following soil acidification. Soil Science & Plant Nutrition 55: 705–714. https://doi.org/10.1111/j.1747-0765.2009.00407.x.
Acknowledgements
This study was supported by the Czech Science Foundation (GACR 19-05791S), the European Commission (Erasmus+ internship) and the CAS within the program of Strategy AV 21, Land save and recovery. The project would not be possible without tight cooperation with the University of Vienna, where pigment analysis took place. We would like to thank companies Palivový Kombinát Ústí s.p. and Sokolovská Uhelná, who provided the boat and chemical data for our project, and Povodí Ohře, a state enterprise, for providing hydrochemical data.
Funding
Czech Science Foundation (GACR 19-05791S), European Commission (Erasmus+ internship) and by the CAS within the program of the Strategy AV 21, Land save and recovery.
Author information
Authors and Affiliations
Contributions
EK wrote the manuscript. EK, MS, MP and KR designed the study. EK, KC, TB, LS and KR collected the data. EK, TB and MS analysed the data. All authors made substantial contributions to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Judit Padisák
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Konopáčová, E., Schagerl, M., Bešta, T. et al. An assessment of periphyton mats using CHEMTAX and traditional methods to evaluate the seasonal dynamic in post-mining lakes. Hydrobiologia 850, 3143–3160 (2023). https://doi.org/10.1007/s10750-023-05243-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05243-1