Abstract
Various studies on population genetics in the Indo-Pacific that include the Coral Triangle region have revealed phylogeographic patterns in marine species populations. However, little is known about the population structure and connectivity pattern among the coral reefs in four seas surrounding Malaysia: the Strait of Malacca (Andaman Sea), the South China Sea, the Sulu Sea, and the Sulawesi Sea. In view of this, we examined the genetic population structure of two invertebrate species: the blue sea star (Linckia laevigata) and the boring clam (Tridacna crocea) from seven localities across Malaysia, including Pulau Layang-Layang in the Spratly Islands. Additional samples of L. laevigata were obtained from the Davao Gulf, the Philippines. The analyses were based on partial mitochondrial cytochrome oxidase I sequences of L. laevigata and T. crocea. Populations of L. laevigata and T. crocea showed departure from neutrality, indicating selection or population expansion. However, a mismatch analysis suggested population expansion. Lack of genetic population structure in L. laevigata (ΦST = − 0.001, P = 0.399) implies high connectivity among the sampled reef sites. There was subtle genetic structuring in populations of T. crocea (ΦST = 0.032, P = 0.009), but AMOVA did not detect any structure in the a priori groupings. The genetic population structure and scale in connectivity are most likely attributed to the life history traits of the sea star and giant clam species, and also to the geological history and seasonal current patterns of the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Coral Triangle is paramount for its diverse marine habitats and species; yet this region is also faced with various threats, giving it high priority for conservation (Burke et al., 2011, 2012). Studies on population genetics and phylogeographic patterns in the Indo-Pacific including the Coral Triangle region (Crandall et al., 2008a; Carpenter et al., 2011; Bowen et al., 2014; Keyse et al., 2014) have been conducted to understand the processes driving and maintaining the extraordinary biodiversity in this region (Avise et al., 1987; Palumbi, 1997; Barber & Bellwood, 2005), and infer the connectivity among coral reefs. Information on population connectivity is essential in formulating effective management plans or designing MPA networks (Cowen et al., 2007; Cowen & Sponaugle, 2009). Different scales of restricted gene flow or barriers to dispersal have been discovered in the Indo-Pacific region (e.g. Barber et al., 2002; Ravago-Gotanco et al., 2007; Gaither et al., 2011; Crandall et al., 2019) and populations that are potentially isolated or dependent on other reef populations may be vulnerable, and thus would need protection tailored to the unique requirements of these reef areas.
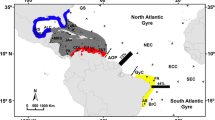
Malaysia is composed of Peninsular Malaysia and East Malaysia separated by the South China Sea. East Malaysia makes up the north-northwest of Borneo and is bounded by the South China Sea in the West and the Sulu Sea and Sulawesi Sea in the East (Fig. 1). The Sulu-Sulawesi seas on the east coast of Sabah mark the boundary of the Coral Triangle for Malaysia, but its exact position there is disputed (Hoeksema, 2007; Veron et al., 2009; Lane & Hoeksema, 2016), and a review by Veron et al. (2015), including new data from the South China Sea (Huang et al., 2015) suggested a westward extension of this biogeographic boundary to include the South China Sea overlying the Sunda Shelf. Few reef areas have been sampled in Malaysia (Gaither & Rocha, 2013) and very little is known about the connectivity patterns across this region of the South China Sea.
Sampling sites across Malaysia: Payar (PAY), Redang (RED), Tioman (TIO), Pulau Tiga (PT), Kota Kinabalu (KK), Kudat (TMP), Semporna (SEM), and Pulau Layang-Layang, Spratly Islands (LAC). An additional locality is the Davao Gulf, the Philippines (DAV). Locality data is given in Table 1. Dark grey shading are present-day land formations; whereas light grey shading are areas exposed during the Last Glacial Maximum (26,000–20,000 years ago) when the sea level stand was >130 m lower than at present (20002007, De Groeve et al., 2022). Dashed arrows indicate seasonal reversing current patterns
For population genetic studies, Linckia laevigata (Linnaeus, 1758) and Tridacna crocea Lamarck, 1819 are two of the top five model species with the highest number of locations sampled across the Indian and Pacific Oceans, and further studies were recommended to include mtDNA COI markers to address the gap in sampling localities (Keyse et al., 2014). Linckia laevigata is common in most coral reef environments from the Western Indian Ocean to south-eastern Polynesia (Clark & Rowe, 1971; Marsh, 1974; Yamaguchi, 1977). Populations from the Indian Ocean and the West Pacific are genetically dissimilar from each other, with the former predominantly being represented by an orange colour morph and the latter by a blue morph (Williams & Benzie, 1998). This sea star reproduces by broadcast spawning, releasing gametes into the water column for external fertilisation (Yamaguchi, 1973) and its larvae have a long pelagic duration of 22–28 days (Yamaguchi, 1977).
Tridacna crocea is the smallest giant clam in the genus Tridacna and can be found in shallow reef environments from the Eastern Indian Ocean to the West Pacific (Othman et al., 2010; Neo et al., 2017). Similar to L. laevigata, this Tridacna species reproduces by broadcast spawning, but has a pelagic larval duration of 7–14 days (Lucas, 1988; Shokita et al., 1991) and is able to settle on the reef in approximately 10 days (Copland & Lucas, 1988) by boring into the reef substrate.
Previous studies on the population structure of L. laevigata and T. crocea indicate varying degrees of gene flow among populations of these species over a wide geographical range (Crandall et al., 2008b; Otwoma & Kochzius, 2016; Hui et al., 2017; Keyse et al., 2018). There has been limited studies of these species in Malaysia. Therefore, in this study we aim to examine the genetic population structure of both species to infer the degree of connectivity among the coral reefs of Malaysia. This information is relevant for finding marine biogeographic boundaries across seas around Malaysia and in particular between sites in- and outside the Coral Triangle, with its westernmost boundary still under debate. Based on the possible westward extension of the Coral Triangle (Huang et al., 2015; Veron et al., 2015) and the course of major currents in the South China Sea (e.g. van der Ven et al., 2021, Fig. 1), we expect to find population connectivity among the South China Sea, Sulu and Sulawesi Seas.
Materials and methods
Study area and sample collection
Linckia laevigata and Tridacna crocea were sampled in the years 2010–2013 from seven localities across Malaysia (Fig. 1, Table 1), including an atoll, Pulau Layang-Layang (LAC), in the southern Spratly Islands (Waheed et al., 2015a). Sampling was carried out within marine parks (Waheed & Hoeksema, 2013, 2014; Waheed et al., 2015b), except for 44 out of 59 sites in Semporna (SEM) and sites in Layang-Layang (LAC). Additional samples of L. laevigata were obtained from the Davao Gulf, the Philippines (DAV). Samples of the bright blue morph of L. laevigata were collected in the form of a piece of arm cutting (~ 2–3 cm). For T. crocea some mantle tissue was collected. All samples were stored in 95% ethanol prior to DNA extraction.
DNA extraction, amplification and sequencing
DNA was isolated from the tissue using the DNeasy Blood and Tissue Kit (QIAGEN) following the standard protocol for animal tissue (version 07/2006). The mitochondrial COI gene was amplified using primers HCO2198 and LCO1490 (Folmer et al., 1994) for L. laevigata, and primers COI-Tricro-Frwd and COI-Tricro-Rev (Kochzius & Nuryanto, 2008) for T. crocea.
The PCR amplification was carried out in a 25 µl reaction volume, which contained 2.5 µl PCR CoralLoad Buffer (containing 15 mM MgCl2) (QIAGEN), 1.0 µl of each primer (10 pmol), 0.5 µl dNTPs (2.5 mM), 0.5 µl Taq polymerase (15 units/ µl) (QIAGEN), 18.5 µl of extra pure PCR water and 1.0 µl diluted DNA extract. The PCR cycles consisted of an initial denaturation step of 94 °C for 3 min, followed by 39 cycles of 94 °C for 1 min, annealing temperature of 5 °C for 1 min, an extension step of 72 °C for 1 min and a final elongation step of 72 °C for 5 min. The amplicons were analysed on a 1% agarose gel electrophoresis, stained with ethidium bromide and visualised on a Red™ Personal Imaging System. Successfully amplified samples were sent to Macrogen Europe for PCR cleaning and bidirectional sequencing on an ABI Automated Sequencher 3730xl.
Sequence alignment and phasing
Sequences were assembled and edited with Sequencher 4.10.1 (Gene Codes Corporation), aligned on the GUIDANCE server using MAFFT algorithm (Penn et al., 2010a, b) and trimmed in BioEdit 7.2.5 (Hall, 1999). The sequences did not contain any gaps or ambiguous positions between base pairs. Protein translations were made in MEGA 6 (Tamura et al., 2013) to ensure that stop codons were not present.
Genetic diversity and historical demography
Genetic diversity indices such as haplotype diversity (h) and nucleotide diversity (π) (Nei & Li, 1979; Nei, 1987) were determined for all populations using Arlequin 3.5.1.3 (Excoffier & Lischer, 2010). Tajima’s D (Tajima, 1989) and Fu’s Fs (Fu, 1997) were calculated to assess the neutrality of the marker. Significant negative values could indicate a selection sweep or recent population expansion, while positive values could reflect balancing selection or a population bottleneck (Tajima, 1989, 1993; Fu & Li, 1993). Fu’s Fs is more sensitive in detecting population growth (Fu, 1997). To further investigate the demographic changes of each species, mismatch distributions were analysed to test the model of sudden population expansion (Slatkin & Hudson, 1991; Rogers & Harpending, 1992; Rogers, 1995). Populations that have undergone growth or expansion are expected to have unimodal and smooth distributions, whereas populations in demographic equilibrium are expected to have multimodal and ragged distributions. Sum of squared deviations (SSD) tests the fit between the observed and expected distribution (Schneider & Excoffier, 1999), whereas Harpending’s raggedness index (HRI) evaluates the smoothness of the distributions (Harpending, 1994). Non-significant SSD and HRI values support a sudden population expansion (Harpending, 1994). In order to obtain accurate estimates of the demographic analyses, only locations with more than five samples were included in the analyses, which then excluded Layang-Layang and Tioman for L. laevigata. Additional analyses were run using only haplogroup A of L. laevigata (Fig. 2) to examine population demographic changes of this haplogroup. All demographic analyses were tested for statistical soundness with 10,000 permutations in Arlequin 3.5.1.3 (Excoffier & Lischer, 2010) with the final significance level determined by sequential Bonferroni corrections (Rice, 1989).
a Haplotype network of Linckia laevigata. Each circle is a unique haplotype. The size of the circle is proportional to the haplotype frequency. The line between the circles represents one mutational step, and hatches represent additional mutational steps. b Distribution of haplogroups across L. laevigata populations
Population structure and gene flow
Population genetic structuring was examined by (1) creating haplotype networks to visualise the relationships among the unique haplotypes, (2) calculating Φ statistics and conducting analysis of molecular variance (AMOVA) to determine the degree of population differentiation among the locations, (3) performing hierarchical AMOVA to identify potential population groupings based on geographical affinities, and (4) determining the association between genetic distance and geographical distance implemented with Mantel test to infer patterns of isolation by distance and gene flow among the populations.
A haplotype network for each species was constructed based on unique haplotypes using the statistical parsimony principle (Templeton et al., 1992) as implemented in TCS version 1.21 (Clement et al., 2000). All haplotypes (of L. laevigata and T. crocea) were included in this analysis. Haplogroups were defined as clusters of haplotypes separated by the highest number of mutational steps (Kochzius et al., 2009). The haplogroups were depicted as pie charts and overlaid onto the map of the study area.
AMOVA was employed to calculate pairwise ΦST-values among all locations (excluding populations with less than five samples, i.e. Layang-Layang and Tioman for L. laevigata). Hierarchical groupings were made by considering the geography of the study site. Hierarchical AMOVA was conducted to calculate ΦST, ΦSC and ΦCT values to assess genetic variation of each grouping and determine the grouping with the most probable partitioning (largest ΦCT difference). Statistical significance was assessed with 10,000 permutations in Arlequin and significance level was Bonferroni adjusted. The analyses were run using the Tamura and Nei model of nucleotide substitution, which is the best approximation of the TIM1 and TPM3uf models (as determined in jModelTest) available in Arlequin 3.5.1.3 (Excoffier & Lischer, 2010). For L. laevigata, a gamma distribution (0.108) was applied based on the most appropriate model of nucleotide substitution determined in jModelTest 2.1.6 (Darriba et al., 2012) based on Akaike Information Criterion (AIC). Additional pairwise ΦST and hierarchical AMOVA analyses were run using only haplogroup A of L. laevigata to account for effects of admixture of the Pacific and Indian Ocean haplogroups (see Crandall et al., 2008b).
For the Isolation by Distance (IBD) analysis, correlation between genetic (pairwise ΦST) and geographical distance (km) was evaluated using Mantel test (Manly, 1994) and reduced major axis (RMA) regression in Isolation by Distance Web Service (IBDWS) version 3.23 (Jensen et al., 2005) with 10,000 randomisations. Geographical distance was measured as the shortest path between two populations by sea, estimated on Google Earth version 7.1.5.1557.
Results
Sampling efforts were performed at 180 sites in seven localities in Malaysia, 18 sites in Layang-Layang and five sites in the Davao Gulf, the Philippines. Both species were absent from two localities: Pulau Payar in the Strait of Malacca, and Pulau Redang, Peninsular Malaysia in the South China Sea. Tridacna crocea was also absent from Kota Kinabalu and Pulau Tiga at the time of the sampling.
Sequence data were obtained for 353 individuals from both species throughout nine sampling localities. Mitochondrial COI fragments of 601 bp from 233 Linckia laevigata and 439 bp from 98 Tridacna crocea resulted in 120 and 51 haplotypes, respectively. All unique haplotypes have been deposited in GenBank (L. laevigata: ON385154-ON385386; T. crocea: ON385440-ON385537).
Genetic diversity and historical demography
Genetic diversity within populations of L. laevigata and T. crocea was almost similar to each other, albeit slightly higher in the former (Table 2). High values of haplotype (h) and nucleotide (π) diversity were recorded (average values of L. laevigata: h = 0.98, π = 1.8; and T. crocea: h = 0.96, π = 1.1). Further analyses were not conducted for L. laevigata from Layang-Layang (n = 3) and Tioman (n = 1) due to the small sample size.
Tajima’s D values were negative for all L. laevigata and T. crocea populations, and all values were not significant (Table 2). Fu’s Fs values were negative for all L. laevigata and T. crocea populations, and only significant in Semporna, Kudat and Kota Kinabalu populations of L. laevigata and Semporna and Layang-Layang populations of T. crocea (P < 0.0001). Haplogroup A of L. laevigata showed similar negative values for Tajima’s D and Fu’s Fs for all populations. Fu’s Fs values were significant in all populations, while Tajima’s D value was significant in the Semporna population only (Supplementary Table S1). The overall negative values for L. laevigata and T. crocea indicate an excess of rare haplotypes compared to what would be expected under neutrality, suggesting selection or population size expansion (Fu, 1997), especially for populations with supporting statistical significance in Fu’s Fs test. The mismatch distributions showed multimodal patterns for L. laevigata with a significant SSD for the Semporna population (SSD = 0.119, P < 0.002), which indicates a constant population size or a declining population size. The non-significant values of HRI and SSD test for the Kudat, Kota Kinabalu, Pulau Tiga and Davao Gulf populations did not match the mismatch distributions under the population expansion model (Supplementary Fig. S1). However, the mismatch distributions for L. laevigata haplogroup A showed unimodal patterns for all populations with a significant SSD and HRI for the Kota Kinabalu population (Supplementary Fig. S2). For T. crocea, raggedness indices from the mismatch distributions were all non-significant. Unimodal curves were observed for Semporna and Layang-Layang populations, supporting the model of recent population expansion for these two locations (Supplementary Fig. S3).
Population structure and gene flow
For L. laevigata, different haplogroups were separated by five or more mutations. Haplotypes from haplogroup A (163 samples) were shared by all populations, and haplogroup B (60 samples) was only present in the populations from north Borneo and the Davao Gulf, whereas haplogroup C (10 samples) occurred exclusively in north Borneo, with a higher frequency in the west coast of Sabah (Fig. 2).
In T. crocea, the two main haplogroups were separated by three mutations. The distribution of the haplogroups on the map showed no clear pattern of subdivision. All sampled populations contained haplogroups A (86 samples) and B (10 samples), whereas the two singletons were from Tioman (C), and Semporna (D) (Fig. 3).
a Haplotype network of Tridacna crocea. Each circle is a unique haplotype. The size of the circle is proportional to the haplotype frequency. The line between the circles represents one mutational step, and hatches represent additional mutational steps. b Distribution of haplogroups across T. crocea populations
Overall genetic structure for L. laevigata was very low (ΦST = − 0.001, P = 0.399) and the pairwise ΦST-values between populations of L. laevigata were also very low, suggesting very little to no population differentiation. Similarly, low pairwise ΦST-values and no population differentiation were observed in populations of L. laevigata from haplogroup A. There was subtle genetic structuring in T. crocea (ΦST = 0.032, P = 0.009) and significant genetic structure was detected between populations of Tioman and Semporna (ΦST = 0.053, P = 0.005), and Tioman and Layang-Layang (ΦST = 0.074, P = 0.006) (Supplementary Table S2).
Hierarchical AMOVA yielded very low support values in all a priori groupings and none were significant after Bonferroni correction. In both species, the percentage of variation within populations was much higher than the variation among populations within regions and among region groupings (Supplementary Table S3). AMOVA confirmed no significant structure in L. laevigata in the region. Furthermore, AMOVA did not detect structure in the assigned groupings for T. crocea, despite the significant pairwise ΦST values between populations of Tioman and Semporna, and populations of Tioman and Layang-Layang.
There was no correlation between genetic and geographical distances across all populations of L. laevigata (r = − 0.142, P = 0.619) and T. crocea (r = 0.067, P = 0.165) as indicated by Mantel test (Supplementary Fig. S4).
Discussion
In this study, sampling efforts were performed at seven localities across Malaysia, but both species were not encountered at two localities: Pulau Redang and Pulau Payar, at the east and west coast of Peninsular Malaysia, respectively. There have been anecdotal reports of L. laevigata from Redang, but no information is available for the Strait of Malacca, where Pulau Payar is located. Nevertheless, this species has been found in the Andaman Sea, situated northward from the Strait of Malacca (Putchakarn & Sonchaeng, 2004). Populations of T. crocea have been reported from the east coast of Peninsular Malaysia (Harborne et al., 2000; Wong & Arshad, 2011) in Pulau Tioman (Tan et al., 1998; Kee Alfian et al., 2005; Neo et al., 2015) and Pulau Redang (Lee et al., 2012), however specimens were only encountered and collected from Tioman. This species has also been reported from Lee Pae Island (= Koh Lipe) and Mu Ko Surin Marine Park in Thailand (Chantrapornsyl et al., 1996), north of Payar. Tridacna crocea was also curiously absent from Kota Kinabalu and Pulau Tiga along the west coast of Sabah, although samples of this species were previously collected from Kota Kinabalu (Kochzius & Nuryanto, 2008).
Genetic variability of L. laevigata and T. crocea were comparable with other populations in the Indo-Malay Archipelago (L. laevigata: Crandall et al., 2008b; Kochzius et al., 2009; Alcazar & Kochzius, 2015, T. crocea: Kochzius & Nuryanto, 2008; DeBoer et al., 2008, 2014b; Neo & Todd, 2012; Hui et al., 2016; Kusnadi et al., 2022), which could suggest a similar demographic history among these population (e.g. Duda & Palumbi, 1999).
Population expansion in L. laevigata was less clear with conflicting outcomes between the neutrality tests and multimodal mismatch distributions in all populations, even though the neutrality tests indicated possible expansion of Semporna, Kudat and Kota Kinabalu. By contrast, the neutrality tests indicated expansion in populations of T. crocea, particularly for Semporna and Layang-Layang and this was further supported by the test of sudden population expansion as well as by the unimodal distribution for these two populations. Population expansion has been observed in populations of T. crocea (Neo & Todd, 2012; DeBoer et al., 2014a; Hui et al., 2016) and L. laevigata in the Indo-Pacific (Crandall et al., 2008b; Kochzius et al., 2009) including the Visayas in the Philippines (Alcazar & Kochzius, 2015).
An almost similar pattern of haplogroups was retrieved for both species with two main haplogroups (A and B) with shared haplotypes among all sampled populations. A smaller haplogroup (C) composed of three haplotypes was primarily found in north Borneo for L. laevigata. Similarly, a haplogroup exclusive to the eastern part of the Visayas, separate from the central Visayas, was detected in the Philippines (Alcazar & Kochzius, 2015). Nevertheless, more samples from other localities are needed to determine the presence of a distinct haplogroup in north Borneo.
Based on pairwise comparisons of populations and hierarchical ANOVA, no significant genetic population structure was detected for L. laevigata (in the whole dataset and the dataset containing only haplogroup A). Populations from north Borneo and the Davao Gulf, spanning across the South China Sea, Sulu Sea and Sulawesi Sea were not significantly different from each other, indicating unrestricted gene flow. Connectivity between the seas is facilitated by north-easterly currents from the South China Sea entering the Sulu Sea through the Balabac Strait during the southwest monsoon between August and October, and the southward currents from the Sulu Sea into the Sulawesi Sea in October. Conversely, currents from the Sulu Sea enter the South China Sea during the northeast monsoon (Wyrtki, 1961; van der Ven et al., 2021; Fig. 1). The seasonally changing currents, possibly coupled with the long pelagic larval duration in L. laevigata, may enable larval dispersal across the reef communities of these seas. In addition, the series of reefs between these seas may facilitate larval dispersal (Kimura & Weiss, 1964; Juinio-Meñez et al., 2003).
Weak but significant genetic population structure was detected in T. crocea, especially between populations of Tioman and Semporna, and Tioman and Layang-Layang. However, there was no significant structure in the a priori groupings. The water circulation pattern along the east coast of Peninsular Malaysia reverses between the two monsoon seasons (Xu and Malanotte-Rizzoli, 2013). During the northeast monsoon a south-westerly current from the northern rim of the sea passes the coast of Vietnam and branches into a small current that turns north-westwards into the Gulf of Thailand, and a major current that flows southwards through the Karimata Strait into the Java Sea (Xu & Malanotte-Rizzoli, 2013), or turns north-easterly along the northwest coast of Borneo (Wyrtki, 1961; Morton & Blackmore, 2001). The current circulation pattern changes direction during the southwest monsoon, driving a northward current from the Karimata Strait through the central South China Sea towards the northern rim of the sea (Wyrtki, 1961; Morton & Blackmore, 2001; Xu & Malanotte-Rizzoli, 2013). This seasonally reversed circulation pattern creates a complex system of eddies and gyres in the South China Sea (Qu, 2000; Tangang et al., 2011; Xu & Malanotte-Rizzoli, 2013), which could aid in retaining or dispersing larvae across the reefs systems in this shallow sea, thereby connecting the reef populations between north Borneo and the east coast of Peninsular Malaysia. For example, Kochzius and Nuryanto (2008) suggested connectivity between T. crocea populations of Kota Kinabalu and Pulau Seribu in the Java Sea. Likewise, in the nearby Bohol Sea, Central Philippines, populations of T. crocea exhibited genetic affinity as a result of prevailing current patterns in the region (Naguit, 2015).
Studies on a larger scale across the Indo-Pacific have revealed that populations of L. laevigata and T. crocea can be divided into three general regions, (1) Eastern Indian Ocean, (2) central Indo-Malay Archipelago, and (3) Western Pacific (Kochzius et al., 2009; Huelsken et al., 2013; Hui et al., 2016, 2017), with distinct divergence between the Indian Ocean and Pacific Ocean (Williams et al., 2002; Crandall et al., 2008b; Otwoma & Kochzius, 2016). The strongest genetic differentiation has been found to the east of Cenderawasih Bay along northeast New Guinea, thus segregating populations from the west: Cenderawasih Bay, Central Indonesia and Indian Ocean, and the east: Western and Central Pacific (Crandall et al., 2014). Our sampling localities within the South China Sea and Sulu and Sulawesi seas are located to the west of Cenderawasih Bay and are nested within the central Indo-Malay Archipelago, hence exhibited a homogenous population with connectivity among the coral reefs, consistent with the previous findings. Simulation models of connectivity structure distinguished the Spratly Islands (South China Sea), Palawan, the Philippines and the Sulu Archipelago as a region with high levels of connectivity, with the Spratly Islands as an upstream source of larvae for the Sulu Archipelago and the Coral Triangle (Kool et al., 2011) as well as other reefs areas within the South China Sea (Dorman et al., 2015). This underscores the importance of the Spratly Islands and the need to designate a conservation zone or have a concerted effort to establish a management regime for these groups of islands (McManus, 1994; Oakley & Pilcher, 1996; Ablan et al., 2002).
Approximately 26,000–20,000 years ago, when the sea level was >130 m lower than at present (De Groeve et al., 2022), Borneo and Palawan were separated by the Balabac Strait, but by a width of only 12 km, while Borneo and Sulawesi were separated by a narrow, deep oceanic trench (Voris, 2000). These narrow pathways may have allowed gene flow between these seas (but see McManus (1985) for review on the intermittent isolation between the South China Sea and Sulu Sea). According to Potts (1983, 1984, 1985) Plio-Pleistocene regressions occurred too frequently and lasted too shortly for speciation and perhaps this may also explain a lack of differentiation at population level for some species.
At a sea level 30 m lower than the present, land mass between Borneo and Sumatra (including Peninsular Malaysia) were very likely separated (Umbgrove, 1947; Voris, 2000; Hoeksema, 2007). At present sea level, an approximately tenfold increase in shallow water habitat since the end of the Last Glacial Maximum around 20,000 years ago has allowed dispersal of marine species across the Sunda Shelf and between the Pacific and Indian Ocean (Bellwood & Wainwright, 2002; Crandall et al., 2012; Gaither & Rocha, 2013). This allows for marine species with moderate to long pelagic larval duration to establish in the Sunda Shelf, such as T. crocea and L. laevigata, albeit at a slower rate in the former than the latter (see Juinio-Meñez et al., 2003, and for other examples see Crandall et al., 2012), and to disperse under the influence of prevailing monsoonal current patterns.
Our results on the lack of genetic structuring in populations of L. laevigata and T. crocea in the South China Sea, Sulu and Sulawesi seas of Malaysia and the Davao Gulf in the Philippines suggest connectivity among the sampled reef populations, which is influenced by seasonal circulation patterns in the seas. Samples for this study in Malaysia were collected from marine park areas except for 44 out of 59 sites in Semporna, which are outside of the Tun Sakaran Marine Park boundary (Waheed & Hoeksema, 2013), and sites in Layang-Layang, the Spratly Islands. Semporna has the largest concentration of coral reefs in Malaysia and is known to have high species richness of hard coral, including a few endemic species (Waheed & Hoeksema, 2013). In spite of this, only small areas have been gazetted as the Tun Sakaran Marine Park, and the Sipadan Island Park. Reefs in Semporna should be assessed to determine appropriate conservation or management measures tailored to address the issues confronting these reefs, such as blast fishing (Kassem et al., 2012). Layang-Layang, together with the other islands and reefs in the Spratly Islands, is very likely an important source of larvae for other populations in the South China Sea, the Sulu Sea and the Coral Triangle, and should be part of a network of MPAs in the region (McManus, 1994; McManus & Meñez, 1997; Huang et al., 2016). As yet, no conservation measures have been afforded to these islands and reefs (McManus, 2017; Ablan-Lagman, 2019; Robles, 2020).
Data availability
Sampling locations and mtCOI sequence files are available in GENBANK.
Code availability
Not applicable.
References
Ablan, M. C. A., J. W. McManus, C. A. Chen, K. T. Shao, J. Bell, A. S. Cabanban, V. S. Tuan & I. W. Arthana, 2002. Meso-scale transboundary units for the management of coral reefs in the South China Sea area. NAGA Rep 25: 4–9.
Ablan-Lagman, M. C., 2019. The Spratly Islands. In Sheppard, C. (ed), World Seas: An Environmental Evaluation: The Indian Ocean to the Pacific, vol. II. Academic Press, London: 583–591.
Alcazar, D. S. R. & M. Kochzius, 2015. Genetic population structure of the blue sea star Linckia laevigata in the Visayas (Philippines). Journal of the Marine Biological Association of the United Kingdom 96: 707–713.
Avise, J. C., J. Arnold, R. M. Ball, E. Bermingham, T. Lamb, J. E. Neigel, C. A. Reeb & N. C. Saunders, 1987. Intraspecific phylogeography: the mitochondrial bridge between population genetics and systematics. Annual Review of Ecology and Systematics 18: 489–522.
Barber, P. H. & D. R. Bellwood, 2005. Biodiversity hotspots: evolutionary origins of biodiversity in wrasses (Halichoeres: Labridae) in the Indo-Pacific and new world tropics. Molecular Phylogenetics and Evolution 35: 235–253.
Barber, P. H., S. R. Palumbi, M. V. Erdmann & M. Kasim Moosa, 2002. Sharp genetic breaks among populations of Haptosquilla pulchella (Stomatopoda) indicate limits to larval transport: patterns, causes, and consequences. Molecular Ecology 11: 659–674.
Bellwood, D. R. & P. C. Wainwright, 2002. The history and biogeography of fishes on coral reefs. In Sale, P. F. (ed), Coral Reef Fishes. Dynamics and Diversity in a Complex Ecosystem. Academic Press, San Diego: 5–32.
Bowen, B. W., K. Shanker, N. Yasuda, M. C. D. Malay, S. von der Heyden, G. Paulay, L. A. Rocha, K. A. Selkoe, P. H. Barber, S. T. Williams, H. A. Lessios, E. D. Crandall, G. Bernardi, C. P. Meyer, K. E. Carpenter & R. J. Toonen, 2014. Phylogeography unplugged: comparative surveys in the genomic era. Bulletin of Marine Science 90: 13–46.
Burke, L., K. Reytar, M. D. Spalding & A. Perry, 2011. Reefs at Risk Revisited, World Resources Institute, Washington.
Burke, L., K. Reytar, M. D. Spalding & A. Perry, 2012. Reefs at Risk Revisited in the Coral Triangle, World Resources Institute, Washington.
Carpenter, K. E., P. H. Barber, E. D. Crandall, M. C. A. Ablan-Lagman, Mahardika GN. Ambariyanto, B. M. Manjaji-Matsumoto, M. A. Juinio-Meñez, M. D. Santos, C. J. Starger & A. H. A. Toha, 2011. Comparative phylogeography of the Coral Triangle and implications for marine management. Journal of Marine Biology 2011: 396982.
Chantrapornsyl, S., K. Kittiwattanawong & K. Adulyanukosol, 1996. Distribution and abundance of giant clam around Lee-Pae Island, the Andaman Sea, Thailand. Phuket Marine Biological Center Special Publication 16: 195–200.
Clark, A. M. & F. W. E. Rowe, 1971. Shallow-water Indo-West-Pacific echinoderms. Trustees of the British Museum (Natural History), London.
Clement, M., D. Posada & K. A. Crandall, 2000. TCS: a computer program to estimate gene genealogies. Molecular Ecology 9: 1657–1659.
Crandall, E. D., M. A. Frey, R. K. Grosberg & P. H. Barber, 2008a. Contrasting demographic history and phylogeographical patterns in two Indo-Pacific gastropods. Molecular Ecology 17: 611–626.
Crandall, E. D., M. E. Jones, M. M. Muñoz, B. Akinronbi, M. V. Erdmann & P. H. Barber, 2008b. Comparative phylogeography of two seastars and their ectosymbionts within the Coral Triangle. Molecular Ecology 17: 276–5290.
Crandall, E. D., E. J. Sbrocco, T. S. DeBoer, P. H. Barber & K. E. Carpenter, 2012. Expansion dating: calibrating molecular clocks in marine species from expansions onto the Sunda Shelf following the Last Glacial Maximum. Molecular Biology and Evolution 29: 707–719.
Crandall, E. D., E. A. Treml, L. Liggins, L. Gleeson, N. Yasuda, P. Barber, G. Wörheide & C. Riginos, 2014. Return of the ghosts of dispersal past: historical spread and contemporary gene flow in the blue sea star Linckia laevigata. Bulletin of Marine Science 90: 399–425.
Crandall, E. D., C. Riginos, C. E. Bird, L. Liggins, E. Treml, M. Beger, et al., 2019. The molecular biogeography of the Indo-Pacific: testing hypotheses with multispecies genetic patterns. Global Ecology and Biogeography 58: 403–418.
Copland, J. W. & J. S. Lucas, 1988. Giant clams in Asia and the Pacific. Australian Center for International Agricultural Research Monograph No. 9, Canberra, Australia.
Cowen, R. K. & S. Sponaugle, 2009. Larval dispersal and marine population connectivity. Annual Review of Marine Science 1: 443–466.
Cowen, R. K., G. Gawarkiewicz, J. Pineda, S. R. Thorrold & F. E. Werner, 2007. Population connectivity in marine systems: an overview. Oceanography 20: 14–21.
Darriba, D., G. L. Taboada, R. Doallo & D. Posada, 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772.
DeBoer, T. S., M. D. Subia, M. V. Erdmann, K. Kovitvongsa & P. H. Barber, 2008. Phylogeography and limited genetic connectivity in the endangered boring giant clam across the Coral Triangle. Conservation Biology 22: 1255–1266.
DeBoer, T. S., M. R. A. Naguit, M. V. Erdmann, M. C. A. Ablanlagman, Carpenter KE. Ambariyanto, A. H. A. Toha & P. H. Barber, 2014a. Concordance between phylogeographic and biogeographic boundaries in the Coral Triangle: conservation implications based on comparative analyses of multiple giant clam species. Bulletin of Marine Science 90: 277–300.
DeBoer, T. S., M. R. A. Naguit, M. V. Erdmann, M. C. A. Ablanlagman, K. E. Carpenter, A. H. A. Toha & P. H. Barber, 2014b. Concordant phylogenetic patterns inferred from mitochondrial and microsatellite DNA in the giant clam Tridacna crocea. Bulletin of Marine Science 90: 301–329.
De Groeve, J., B. Kusumoto, E. Koene, W. D. Kissling, A.C. Seijmonsbergen, B.W. Hoeksema, B.W., et al., 2022. Global raster dataset on historical coastline positions and shelf sea extents since the Last Glacial Maximum. Global Ecology and Biogeography 31: 2162–2171.
Dorman, J. G., F. S. Castruccio, E. N. Curchitser, J. A. Kleypas & T. M. Powell, 2015. Modeled connectivity of Acropora millepora populations from reefs of the Spratly Islands and the greater South China Sea. Coral Reefs 35: 169–179.
Duda, T. F., Jr. & S. R. Palumbi, 1999. Population structure of the black tiger prawn Panaeus monodon, among western Indian Ocean and western Pacific populations. Marine Biology 134: 705–710.
Excoffier, L. & H. E. L. Lischer, 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10: 564–567.
Folmer, O., M. Black, W. R. Hoeh, R. Lutz & R. C. Vrijenhoek, 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
Fu, Y. X., 1997. Statistical tests of neutrality against population growth, hitchhiking and background selection. Genetics 147: 915–925.
Fu, Y. X. & W. H. Li, 1993. Statistical tests of neutrality of mutations. Genetics 133: 693–707.
Gaither, M. R. & L. A. Rocha, 2013. Origins of species richness in the Indo-Malay-Philippine biodiversity hotspot: evidence for the centre of overlap hypothesis. Journal of Biogeography 40: 1638–1648.
Gaither, M. R., B. W. Bowen, T.-R. Bordenave, L. A. Rocha, S. J. Newman, J. A. Gomez, L. van Herwerden & M. T. Craig, 2011. Phylogeography of the reef fish Cephalopholus argus (Epinephelidae) indicates Pleistocene isolation across the Indo-Pacific Barrier with contemporary overlap in the Coral Triangle. BMC Evolutionary Biology 11: 189.
Google Earth, 2015. Version 7.1.5.1557. https://www.google.com/earth/.
Hall, T. A., 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98.
Harborne, A., D. Fenner, A. Barnes, M. Beger, S. Harding & T. Roxburgh, 2000. Status Report on the Coral Reefs of the East Coast of Peninsula Malaysia, Coral Cay Conservation Ltd, London.
Harpending, H., 1994. Signature of ancient population growth in slow-resolution mitochondrial DNA mismatch distribution. Human Biology 66: 591–600.
Hoeksema, B. W., 2007. Delineation of the Indo-Malayan centre of maximum marine biodiversity: the Coral Triangle. In Renema, W. (ed), Biogeography, Time and Place: Distributions, Barriers and Islands. Springer, Dordrecht: 117–178.
Huang, D., W. Y. Licuanan, B. W. Hoeksema, C. A. Chen, P. O. Ang, H. Huang, D. J. Lane, S. T. Vo, Z. Waheed, Y. A. Affendi & T. Yeemin, 2015. Extraordinary diversity of reef corals in the South China Sea. Marine Biodiversity 45: 157–168.
Huang, D., B. W. Hoeksema, Y. A. Affendi, P. O. Ang, C. A. Chen, H. Huang, D. J. W. Lane, W. Y. Licuanan, O. Vibol, S. T. Vo, T. Yeemin & L. M. Chou, 2016. Conservation of reef corals in the South China Sea based on species and evolutionary diversity. Biodiversity and Conservation 25: 331–344.
Huelsken, T., J. Keyse, L. Liggins, S. Penny, E. A. Treml & C. Riginos, 2013. A novel widespread cryptic species and pylogeographic patterns within several giant clam species (Cardiidae: Tridacna) from the Indo-Pacific Ocean. PLoS ONE 8: e80858.
Hui, M., W. E. Kraemer, C. Seidel, A. Nuryanto, A. Joshi & M. Kochzius, 2016. Comparative genetic population structure of three endangered giant clams (Cardiidae: Tridacna species) throughout the Indo-West Pacific: implications for divergence, connectivity and conservation. Journal of Molluscan Studies 82: 403–414.
Hui, M., A. Nuryanto & M. Kochzius, 2017. Concordance of microsatellite and mitochondrial DNA markers in detecting genetic population structure in the boring giant clam Tridacna crocea across the Indo-Malay Archipelago. Marine Ecology 38: e12389.
Jensen, J. L., A. J. Bohonak & S. T. Kelley, 2005. Isolation by distance, web service. Ver. 3.23 BMC Genetics 6:13. http://ibdws.sdsu.edu/.
Juinio-Meñez, M. A., R. M. Magsino, R. Ravago-Gotanco & E. T. Yu, 2003. Genetic structure of Linckia laevigata and Tridacna crocea populations in the Palawan shelf and shoal reefs. Marine Biology 142: 717–726.
Kassem, K., B. Hoeksema & Y. A. Affendi (eds), 2012. Semporna Marine Ecological Expedition. WWF-Malaysia, NCB Naturalis, Universiti Malaysia Sabah, Kota Kinabalu.
Kee Alfian, A. A., W. S. Wong, H. Badrul & Y. A. Affendi, 2005. Macroinvertebrates diversity of Kg. Tekek, Pulau Tioman Marine Park. Proceedings of Second Regional Symposium on Environment and Natural Resources, Vol. 2. 22–23 March 2005, Pan Pacific Hotel, Kuala Lumpur, Malaysia: 79–84.
Keyse, J., E. D. Crandall, R. J. Toonen, C. P. Meyer, E. A. Treml & C. Riginos, 2014. The scope of published population genetic data for Indo-Pacific marine fauna and future research opportunities in the region. Bulletin of Marine Science 90: 47–78.
Keyse, J., E. A. Treml, T. Huelsken, P. H. Barber, T. DeBoer, M. Kochzius, A. Nuryanto, J. P. A. Gardner, L. L. Liu, S. Penny & C. Riginos, 2018. Historical divergences associated with intermittent land bridges overshadow isolation by larval dispersal in co-distributed species of Tridacna giant clams. Journal of Biogeography 45: 848–858.
Kimura, M. & G. H. Weiss, 1964. The stepping stone model of population structure and the decrease of genetic correlation with distance. Genetics 49: 561–576.
Kochzius, M. & A. Nuryanto, 2008. Strong genetic population structure in the boring giant clam, Tridacna crocea, across the Indo-Malay Archipelago: implications related to evolutionary processes and connectivity. Molecular Ecology 17: 3775–3787.
Kochzius, M., C. Seidel, J. Hauschild, S. Kirchhoff, P. Mester, I. Meyer-Wachsmuth, A. Nuryanto & J. Timm, 2009. Genetic population structure of the blue starfish Linckia laevigata and its gastropod ectoparasite Thyca crystallina. Marine Ecology Progress Series 396: 211–219.
Kool, J. T., C. B. Paris, P. H. Barber & R. K. Cowen, 2011. Connectivity and the development of population genetic structure in Indo-West Pacific coral reef communities. Global Ecology and Biogeography 20: 695–706.
Kusnadi, A., D. Kurnianto, H. Madduppa, N. P. Zamani, P. S. Ibrahim, E. HernawanU, R. T. Utami & T. Triandiza, 2022. Genetic diversity and population structure of the boring giant clam (Tridacna crocea) in Kei Islands, Maluku, Indonesia. Biodiversitas 23: 1273–1282.
Lamarck, J.-B. M. de, 1819. Histoire naturelle des animaux sans vertèbres. Tome sixième, 1re partie. Paris: published by the Author.
Lane, D. J. W. & B. W. Hoeksema, 2016. Mesophotic mushroom coral records at Brunei Darussalam support westward extension of the coral triangle to the South China Sea waters of Northwest Borneo. Raffles Bulletin of Zoology 64: 204–212.
Lee, J. N., S. Mohd, M. N. Ismail & B. A. Norhayati, 2012. Carrying capacity of Pulau Redang Marine Park 2011. Department of Marine Parks Malaysia, Putrajaya.
Linnaeus, C., 1758. Tomus I. Systema Naturae. Holmiae, Laurentii Salvii.
Lucas, J. S., 1988. Giant clams: description, distribution and life history. In Copland, J. W. & J. S. Lucas (eds), Giant Clams in Asia and the Pacific Australian Centre for International Agricultural Research, Canberra: 21–32.
Manly, B. F. J., 1994. Multivariate Statistical Methods: A Primer, 2nd ed. Chapman and Hall, New York.
Marsh, L. M., 1974. Shallow-water asterozoans of southeastern Polynesia. I. Asteroidea. Micronesica 10: 65–104.
McManus, J. W., 1985. Marine speciation, tectonics and sea-level changes in Southeast Asia. Proc 5th Int Coral Reef Symp, Vol. 4: 133–138.
McManus, J. W., 1994. The Spratly Islands: a marine park? Ambio 23: 181–186.
McManus, J. W., 2017. Offshore coral reef damage, overfishing, and paths to peace in the South China Sea. Marine and Coastal Law 32: 199–237.
McManus, J. W. & L. A. B. Meñez, 1997. The proposed international Spratly Island marine park: ecological considerations. Proc 8th Int Coral Reef Symp, Vol. 2: 1943–1948.
Morton, B. & G. Blackmore, 2001. South China Sea. Marine Pollution Bulletin 42: 1236–1263.
Naguit, M. R. A., 2015. Genetic patterns of Tridacna crocea in the Bohol Sea. NMSCST Research Journal 3(1): 26–43.
Nei, M., 1987. Molecular Evolutionary Genetics, Columbia University Press, New York.
Nei, M. & W.-H. Li, 1979. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proceedings of National Academy of Sciences of the United States of America 76: 5269–5273.
Neo, M. L. & P. A. Todd, 2012. Population density and genetic structure of the giant clams Tridacna crocea and T. squamosa on Singapore’s reefs. Aquatic Biology 14: 265–275.
Neo, M. L., W. Eckman, K. Vicentuan, S.L.-M. Teo & P. A. Todd, 2015. The ecological significance of giant clams in coral reef ecosystems. Biology Conservation 181: 111–123.
Neo, M. L., C. C. C. Wabnitz, R. D. Braley, G. A. Heslinga, C. Fauvelot, S. V. Wynsberge, S. Andrefouet, C. Waters, A. S. H. Tan, E. Gomez, M. J. Costello & P. Todd, 2017. Giant clams (Bivalvia: Cardiidae: Tridacninae): a comprehensive update of species and their distribution, current threats and conservation status. Oceanography Marine and Biology 55: 87–387.
Oakley, S. & N. Pilcher, 1996. Marine Protected Areas for sustainable fisheries management: Layang Layang reef as a source of larvae in the South China Sea. Proceedings of Workshop on Aquaculture and Sustainable reef fisheries, Kota Kinabalu, Sabah Malaysia, 4–8th December 1996.
Othman, A. S., G. H. S. Goh & P. Todd, 2010. The distribution and status of giant clams (Family Tridacnidae)—a short review. Raffles Bulletin of Zoology 58: 103–111.
Otwoma, L. M. & M. Kochzius, 2016. Genetic population structure of the coral reef sea star Linckia laevigata in the Western Indian Ocean and Indo-West Pacific. PLoS ONE 11(10): e0165552.
Palumbi, S. R., 1997. Molecular biogeography of the Pacific. Coral Reefs 16: S47–S52.
Penn, O., E. Privman, H. Ashkenazy, G. Landan, D. Graur & T. Pupko, 2010a. GUIDANCE: a web server for assessing alignment confidence scores. Nucleic Acids Research 38: W23–W28.
Penn, O., E. Privman, G. Landan, D. Graur & T. Pupko, 2010b. An alignment confidence score capturing robustness to guide-tree uncertainty. Molecular Biology and Evolution 27: 1759–1767.
Potts, D. C., 1983. Evolutionary disequilibrium among Indo-Pacific corals. Bulletin of Marine Science 33: 619–632.
Potts, D. C., 1984. Generation times and the Quaternary evolution of reef-building corals. Paleobiology 10: 48–58.
Potts, D. C., 1985. Sea-level fluctuations and speciation in Scleractinia. Proc 5th Int Coral Reef Congr, Vol. 4: 127–132.
Putchakarn, S. & P. Sonchaeng, 2004. Echinoderm fauna of Thailand. History and Inventory Reviews. Science Asia 30: 417–428.
Qu, T., 2000. Upper-layer circulation in the South China Sea. Journal of Physics Oceanography 30: 1450–1460.
Ravago-Gotanco, R. G., R. M. Magsino & M. A. Juinio-Menez, 2007. Influence of the North Equatorial Current on the population genetic structure of Tridacna crocea (Mollusca: Tridacnidae) along the eastern Philippine seaboard. Marine Ecology Progress Series 336: 161–168.
Rice, W. R., 1989. Analyzing tables of statistical tests. Evolution 43: 223–225.
Robles, A. C., 2020. Endangered Species and Fragile Ecosystems in the South China Sea. La Salle University Manila, and Palgrave Macmillan, Singapore.
Rogers, A. R., 1995. Genetic evidence for Pleistocene population expansion. Evolution 49: 608–615.
Rogers, A. R. & H. Harpending, 1992. Population growth makes waves in the distribution of pairwise genetic differences. Molecular Biology and Evolution 9: 552–569.
Schneider, S. & L. Excoffier, 1999. Estimation of past demographic parameters from distribution of pairwise differences when the mutation rates vary among sites: application to human mitochondrial DNA. Genetics 152: 1079–1089.
Slatkin, M. & R. R. Hudson, 1991. Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129: 555–562.
Shokita, S., K. Kakazu, A. Tomori & T. Toma, 1991. Aquaculture in tropical areas. Midoro Shobo, Tokyo.
Tajima, F., 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595.
Tajima, F., 1993. Measurement of DNA polymorphism. In Takahata, N. & A. G. Clark (eds), Mechanisms of Molecular Evolution. Introduction to Molecular Paleopopulation Biology Scientific Societies Press, Sinauer Associates Inc, Tokyo: 37–59.
Tamura, K., G. Stecher, D. Peterson, A. Filipski & S. Kumar, 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729.
Tan, A. S. H., Z. B. Yasin, I. B. Salleh & A. A. Yusof, 1998. Status of giant clams in Pulau Tioman, Malaysia. Malayan Nature Journal 52: 205–216.
Tangang, F., C. Xia, F. Qiao, L. Juneng & F. Shan, 2011. Seasonal circulations in the Malay Peninsula Eastern Continental Shelf from a wave-tide-circulation coupled model. Ocean Dynamics 61: 1317–1328.
Templeton, A. R., K. A. Crandall & C. F. Sing, 1992. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram Estimation. Genetics 132: 619–633.
Umbgrove, J. H. F., 1947. Coral reefs of the East Indies. Geological Society of America Bulletin 58: 729–778.
van der Ven, R. M., H. Heynderickx & M. Kochzius, 2021. Differences in genetic diversity and divergence between brooding and broadcast spawning corals across two spatial scales in the Coral Triangle region. Marine Biology 168: 17.
Veron, J. E. N., L. M. DeVantier, E. Turak, A. L. Green, S. Kininmonth, M. G. Stafford-Smith & N. Peterson, 2009. Delineating the Coral Triangle. Galaxea, Journal of Coral Reef Studies 11: 91–100.
Veron, J., M. Stafford-Smith, L. DeVantier & E. Turak, 2015. Overview of distribution patterns of zooxanthellate Scleractinia. Frontiers in Marine Science 1: 81.
Voris, H. K., 2000. Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. Journal of Biogeography 27: 1153–1167.
Waheed, Z. & B. W. Hoeksema, 2013. A tale of two winds: species richness patterns of reef corals around the Semporna peninsula, Malaysia. Marine Biodiversity 43: 37–51.
Waheed, Z. & B. W. Hoeksema, 2014. Diversity patterns of scleractinian corals at Kota Kinabalu, Malaysia, in relation to exposure and depth. Raffles Bulletin Zoology 62: 66–82.
Waheed, Z., F. Benzoni, S. E. van der Meij, T. I. Terraneo & B. W. Hoeksema, 2015a. Scleractinian corals (Fungiidae, Agariciidae and Euphylliidae) of Pulau Layang-Layang, Spratly Islands, with a note on Pavona maldivensis (Gardiner, 1905). ZooKeys 517: 1–37.
Waheed, Z., H. G. J. van Mil, M. A. Syed Hussein, R. Jumin, B. Golam Ahad & B. W. Hoeksema, 2015b. Coral reefs at the northernmost tip of Borneo: an assessment of scleractinian species richness patterns and benthic reef assemblages. PLoS ONE 10: e0146006.
William, S. T. & J. A. H. Benzie, 1998. Evidence of a biogeographic break between populations of a high dispersal starfish: congruent regions within the Indo-West Pacific defined by color morphs, mtDNA, and allozyme data. Evolution 52: 87–99.
Williams, S. T., J. Jara, N. Gomez & N. Knowlton, 2002. The marine Indo-West Pacific break: contrasting the resolving power of mitochondrial and nuclear genes. Integrative Computational Biology 42: 941–952.
Wong, N. L. W. S. & A. Arshad, 2011. A brief review on marine shelled mollusca (Gastropoda and Bivalvia) records in Malaysia. Journal of Fisheries and Aquatic Science 6: 669–699.
Wyrtki, K., 1961. Physical oceanography of the Southeast Asian waters. Naga Report 2, Scripps Institution of Oceanography, San Diego.
Xu, D. & P. Malanotte-Rizzoli, 2013. The seasonal variation of the upper layers of the South China Sea (SCS) circulation and the Indonesian through flow (ITF): an ocean model study. Dynamics of Atmospheric and Oceans 63: 103–130.
Yamaguchi, M., 1973. Early life histories of coral reef asteroids, with special reference to Acanthaster planci (L.). In Jones, O. A. & R. Endean (eds), Biology and Geology of Coral Reefs Academic Press, New York and London: 369–387.
Yamaguchi, M., 1977. Population structure, spawning and growth of the coral reef asteroid Linckia laevigata (Linneaus). Pacific Science 31: 13–30.
Acknowledgements
Research permits for Malaysia were granted at the national level by the Economic Planning Unit and the Malaysian National Security Council under the Prime Minister’s Department, Malaysia, and at the local level by various departments: the Sabah Biodiversity Centre, Sabah Parks, Department of Fisheries Sabah and the Marine Park and Resources Management Division under the Department of Fisheries Malaysia. Samples from Semporna were collected during the Semporna Marine Ecological Expedition (SMEE) 2010, whereas samples from Kudat were collected during the Tun Mustapha Park Expedition (TMPE) 2012. Both expeditions were co-organised by WWF Malaysia, Universiti Malaysia Sabah and Naturalis Biodiversity Center, The Netherlands. Travel expenses for Z. Waheed were funded by the A.M. Buitendijkfonds and the Treub-Maatschappij (The Society for the Advancement of Research in the Tropics), The Netherlands for fieldwork in Layang-Layang and Peninsular Malaysia, respectively. We are grateful to the various dive operators for diving logistics throughout this research: Avillion Layang-Layang, East Marine Holidays Sdn Bhd, Tioman Dive Centre, East Divers Tioman and Redang Kalong Resort. We thank Bastian T. Reijnen for assistance in the laboratory and data analyses. We also thank the editor and two anonymous reviewers for their constructive comments.
Funding
Travel expenses for Z. Waheed were funded by the A.M. Buitendijkfonds and the Treub-Maatschappij (The Society for the Advancement of Research in the Tropics), The Netherlands for fieldwork in Layang-Layang and Peninsular Malaysia, respectively.
Author information
Authors and Affiliations
Contributions
ZW, MK and BWH conceived the ideas; ZW, AB and BWH collected the samples; ZW analysed the samples; ZW analysed the data; and ZW and BWH led the writing with contributions of all the other authors.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study have been followed and all necessary approvals have been obtained. This study complied with all CITES regulations and the necessary permits.
Consent to participate
Consent on behalf of all the authors.
Consent for publication
Consent on behalf of all the authors.
Additional information
Handling editor: Cecile Fauvelot
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Waheed, Z., Bos, A.R., Kochzius, M. et al. Genetic population structure of the blue sea star (Linckia laevigata) and the boring giant clam (Tridacna crocea) across Malaysia. Hydrobiologia 850, 81–95 (2023). https://doi.org/10.1007/s10750-022-05044-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05044-y