Abstract
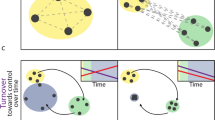
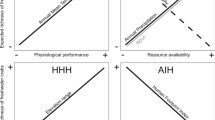
Water bodies vary widely in terms of environmental stability, and most of this variation is due to water permanence. Permanent systems are expected to have a more stable community since the organisms will not be subjected to the severe disturbances caused by droughts. Temporary habitats are stressed by frequent dry phases, which reset the system and trigger succession processes that can drive changes in species composition. Previous studies have found higher beta diversity among permanent than among temporary water bodies, whereas others have found the opposite. We conducted a meta-analysis to compare the beta diversity of aquatic invertebrates in permanent and temporary water bodies and assess whether there is a predominant relationship between beta diversity and water permanence. Our results revealed that the beta diversity of invertebrates did not differ between permanent and temporary habitats. Primary studies showed both patterns: some of them presented higher beta diversity in permanent than in temporary water bodies, whereas others presented the opposite, resulting in a net effect of no difference. The lack of a clear pattern may result from the interaction with multiple factors, including hydrological specificities of the study areas, scales, and dispersal abilities of the organisms investigated, as well as environmental and anthropogenic factors.




Similar content being viewed by others
Data availability
The database used is available in the Supplementary Material section.
References
Anderson, M. J., K. E. Ellingsen & B. H. McArdle, 2006. Multivariate dispersion as a measure of beta diversity. Ecology Letters 9(6): 683–693.
Annani, F., A. H. Alfarhan & B. Samraoui, 2012. Aquatic Hemiptera of northeastern Algeria: distribution, phenology and conservation. Revue D’écologie 67: 1–13.
Anton-Pardo, M. & X. Armengol, 2012. Effects of salinity and water temporality on zooplankton community in coastal Mediterranean ponds. Estuarine, Coastal and Shelf Science 114: 93–99.
Anton-Pardo, M., J. C. Ortega, A. S. Melo & L. M. Bini, 2019. Global meta-analysis reveals that invertebrate diversity is higher in permanent than in temporary lentic water bodies. Freshwater Biology 64: 2234–2246.
Araújo, L. R., P. M. Lopes, J. M. Santangelo, A. C. Petry & R. L. Bozelli, 2013. Zooplankton resting egg banks in permanent and temporary tropical aquatic systems. Acta Limnologica Brasiliensia 25: 235–245.
Arnell, N. W., 1999. Climate change and global water resources. Global Environmental Change 9: S31–S49.
Attayde, J. L. & R. L. Bozelli, 1998. Assessing the indicator properties of zooplankton assemblages to disturbance gradients by canonical correspondence analysis. Canadian Journal of Fisheries and Aquatic Sciences 55: 1789–1797.
Baber, M. J., K. Fleishman, J. Babbit & T. L. Tarr, 2004. The relationship between wetland hydroperiod and nestedness patterns in assemblages of larval amphibians and predatory macroinvertebrates. Oikos 107: 16–27.
Batzer, D. P., C. R. Pusateri & R. Vetter, 2000. Impacts of fish predation on marsh invertebrates: direct and indirect effects. Wetlands 20: 307–312.
Bell, G., 2017. Evolutionary rescue. Annual Review of Ecology, Evolution, and Systematics 48: 605–627.
Boersma, K. S., A. Nickerson, C. D. Francis & A. M. Siepielski, 2016. Climate extremes are associated with invertebrate taxonomic and functional composition in mountain lakes. Ecology and Evolution 6: 8094–8106.
Borenstein, M., L. V. Hedges, J. P. T. Higgins & H. R. Rothstein, 2009. Introduction to Meta-analysis, Wiley, Chichester.
Borenstein, M., J. P. T. Higgins, L. V. Hedges & H. R. Rothstein, 2017. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Research Synthesis Methods 8: 5–18.
Boronat, L., M. R. Miracle & X. Armengol, 2001. Cladoceran assemblages in a mineralization gradient. Hydrobiologia 442: 75–88.
Brendonck, L. & L. DeMeester, 2003. Egg banks in freshwater zooplankton: Evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84.
Campbell, D., P. Humphries, N. McCasker & D. Nielsen, 2018. Subfossil chironomid head capsules reveal assemblage differences in permanent and temporary wetlands of south-eastern Australia. Hydrobiologia 809: 91–110.
Carchini, G., V. D. Bella, A. G. Solimini & M. Bazzanti, 2007. Relationships between the presence of Odonate species and environmental characteristics in Iowland ponds of central Italy. International Journal of Limnology 43(2): 81–87.
Chase, J. M., 2007. Drought mediates the importance of stochastic community assembly. Proceedings of the National Academy of Sciences of the United States of America 104: 17430–17434.
Chase, J. M., 2010. Stochastic community assembly causes higher biodiversity in more productive environments. Science 328: 1388–1391.
Chase, J. M. & T. M. Knight, 2003. Drought-induced mosquito outbreaks in wetlands. Ecology Letters 6: 1017–1024.
Daniel, J., J. E. Gleason, K. Cottenie & R. C. Rooney, 2019. Stochastic and deterministic processes drive wetland community assembly across a gradient of environmental filtering. Oikos 128: 1158–1169.
Datry, T., K. Fritz & C. Leigh, 2016. Challenges, developments and perspectives in intermittent river ecology. Freshwater Biology 61: 1171–1180.
De los Ríos, P., 2008. A null model for explain crustacean species associations in Central and Southern Patagonian inland waters. Anales Del Instituto De La Patagonia 36(1): 25–33.
De los Ríos, P., A. Mansilla & M. Vega, 2010. Species co-occurrences based on a presence/absence null model for copepoda and cladocerans in Patagonia and Tierra del Fuego lakes and ponds. Biologia, Bratislava 65(6): 1019–1027.
De los Ríos, P., G. Figueroa-Muñoz & L. Parra-Coloma, 2018. Null models for explaining inland water crustacean zooplankton communities in Chile. Animal Biology 68: 161–172.
De Meester, L., J. Vanoverbeke, L. J. Kilsdonk & M. C. Urban, 2016. Evolving perspectives on monopolization and priority effects. Trends Ecology and Evolution 31: 136–146.
Devercelli, M., P. Scarabotti, G. Mayora, B. Schneider & F. Giri, 2016. Unravelling the role of determinism and stochasticity in structuring the phytoplanktonic metacommunity of the Paraná River floodplain. Hydrobiologia 764: 139–156.
Diehl, S., 1992. Fish predation and benthic community structure: the role of omnivory and habitat complexity. Ecology 73: 1646–1661.
Drenner, S. M., 2008. Crustacean zooplankton community structure in temporary and permanent ponds in a Texas grassland. Dissertation, Texas Christian University.
Duval, S. & R. Tweedie, 2000. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 53: 455–463.
Elías-Gutiérrez, M., A. A. Kotov & T. Garfias-Espejo, 2006. Cladocera (Crustacea: Ctenopoda, Anomopoda) from southern Mexico, Belize and northern Guatemala, with some biogeographical notes. Zootaxa 1119: 1–27.
Galatowitsch, M. L. & A. R. McIntosh, 2016. Developmental constraints control generalist invertebrate distributions across a gradient of unpredictable disturbance. Freshwater Science 35: 1300–1311.
Galindo, M. D., A. J. Mata, N. Mazuelos & L. Serrano, 1994. Microcrustacean and rotifer diversity and richness relating to water temporality in dune ponds of the Doñana National Park (SW Spain). Internationale Vereinigung Für Theoretische Und Angewandte Limnologie 25: 1350–1356.
Gerstner, K., D. Moreno-Mateos, J. Gurevitch, M. Beckmann, S. Kambach, H. P. Jones & R. Seppelt, 2017. Will your paper be used in a meta-analysis? Make the reach of your research broader and longer lasting. Methods in Ecology and Evolution 8: 777–784.
Gilbert, J. D., I. De Vicente, F. Ortega, R. Jiménez-Melero, G. Parra, F. Guerrero & J. Ruiz, 2015. A comprehensive evaluation of the crustacean assemblages in southern Iberian Mediterranean wetlands. Journal of Limnology 2015(73): 169–181.
Gilinsky, E., 1984. The role of fish predation and spatial heterogeneity in determining benthic community structure. Ecology 65: 455–468.
Gray, L. J., 1981. Species composition and life histories of aquatic insects in a lowland Sonoran Desert stream. The American Midland Naturalist 106: 229–242.
Gyllström, M. & L. A. Hansson, 2004. Dormancy in freshwater zooplankton: Induction, termination and the importance of benthic-pelagic coupling. Aquatic Sciences 66: 274–295.
Hairston, N. G., 1996. Zooplankton egg banks as biotic reservoirs in changing environments. Limnology Oceanography 41: 1087–1092.
Hansen, B. W., 2019. Copepod embryonic dormancy: “an egg is not just an egg.” Biological Bulletin 237: 145–169.
Heino, J., A. S. Melo, T. Siqueira, J. Soininen, S. Valanko & L. M. Bini, 2015. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater. Biology 60: 845–869.
Iglikowska, A. & T. Namiotko, 2012. The impact of environmental factors on diversity of Ostracoda in freshwater habitats of Subartic and temperate Europe. Annales Zoologici Fennici 49: 193–218.
IPCC, 2021. Climate Change 2021: The Physical Science Basis. In MassonDelmotte, V., P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, O. Yelekçi, R. Yu and & B. Zhou (eds.) ]Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. In Press.
Junk, W., 2013. Current state of knowledge regarding South America wetlands and their future under global climate change. Aquatic Sciences 75: 113–131.
Kundzewicz, Z. W., L. J. Mata, N. W. Arnell, P. Döll, B. Jimenez, K. Miller, T. Oki, Z. Şen & I. Shiklomanov, 2008. The implications of projected climate change for freshwater resources and their management. Hydrological Sciences Journal 53: 3–10.
Larned, S. T., T. Datry, D. B. Arscott & K. Tockner, 2010. Emerging concepts in temporary-river ecology. Freshwater Biology 5: 717–738.
Leigh, C. & T. Datry, 2017. Drying as a primary hydrological determinant of biodiversity in river systems: a broad-scale analysis. Ecography 40: 487–499.
Light, R. J. & D. B. Pillemer, 1984. Summing Up: The Science of Reviewing Research, Harvard University Press, Cambridge:
Lopes, P. M., L. M. Bini, S. A. J. Declerck, et al., 2014. Correlates of zooplankton beta diversity in tropical lake systems. PLoS ONE 9: e109581.
Mabidi, A., M. S. Bird, R. Perissinotto & D. C. Rogers, 2016. Ecology and distribution of large branchiopods (Crustacea, Branchiopoda, Anostraca, Notostraca, Laevicaudata, Spinicaudata) of the Eastern Cape Karoo, South Africa. ZooKeys 618: 15–38.
Marrone, F., G. Alfonso, F. Stoch, V. Pieri, M. Alonso, M. Dretakis & L. Naselli-Flores, 2019. An account on the non-malacostracan crustacean fauna from the inland waters of Crete, Greece, with the synonymization of Arctodiaptomus piliger Brehm, 1955 with Arctodiaptomus alpinus (Imhof, 1885). Limnetica 38: 167–187.
Martínez-García, B., O. Suarez-Hernando, J. Mendicoa & X. Murelaga, 2015. Living ostracod species from permanent and semi-permanent ponds of Bardenas Reales de Navarra (Northern Spain) with remarks on their ecological requirements. Ameghiniana 52(6): 598–612.
McCauley, S. J., C. J. Davis, R. A. Relyea, K. L. Yurewicz, D. K. Skelly & E. E. Werner, 2008. Metacommunity patterns in larval odonates. Oecologia 158: 329–342.
Moher, D., A. Liberati, J. Tetzlaff, D. G. Altman, Altman Prisma Group, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 6(7): e1000097.
Oksanen, J. R. Kindt, P, Legendre, B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens & H. Wagner, 2017. vegan: Community Ecology Package. Ordination Methods, Diversity Analysis and Other Functions for Community and Vegetation Ecologists. R Package Version 2.4.4.
Olden, J. D., 2006. Biotic homogenization: a new research agenda for conservation biogeography. Journal of Biogeography 33(12): 2027–2039.
Picazo, F., J. L. Moreno & A. Millán, 2010. The contribution of standing waters to aquatic biodiversity: the case of water beetles in southeastern Iberia. Aquatic Ecology 44: 205–216.
Pinceel, T., F. Buschke, M. Weckx, L. Brendonck & B. Vanschoenwinkel, 2018. Climate change jeopardizes the persistence of freshwater zooplankton by reducing both habitat suitability and demographic resilience. BMC Ecology 18: 2.
Pires, M. M., C. Stenert & L. Maltchik, 2017. Partitioning beta-diversity through different pond hydroperiod lengths reveals predominance of nestedness in assemblages of immature odonates. Entomological Science 20: 318–326.
Poff, N. L., 1997. Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. Journal of the North American Benthological Society 16: 391–409.
R Core Team, 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Reid, A. J., A. K. Carlson, I. F. Creed, et al., 2019. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews 94: 849–873.
Rosset, V., A. Ruhi, M. T. Bogan & T. Datry, 2017. Do lentic and lotic communities respond similarly to drying? Ecosphere 8(7): e01809.
Sahuquillo, M. & M. R. Miracle, 2019. Rotifer communities in Mediterranean ponds in eastern Iberian Peninsula: abiotic and biotic factors defining pond types. Limnetica 38(1): 103–117.
Schell, J. M., C. J. Santos-Flores, P. E. Allen, et al., 2001. Physical-chemical influences on vernal zooplankton community structure in small lakes and wetlands of Wisconsin, U.S.A. Hydrobiologia 445: 37–50.
Schriever, T. A. & D. A. Lytle, 2016. Convergent diversity and trait composition in temporary streams and ponds. Ecosphere 7(5): e01350.
Schultz, T. D., 2009. Diversity and habitats of a prairie assemblage of Odonata at Lostwood National Wildlife Refuge, North Dakota. Journal of the Kansas Entomological Society 82: 91–102.
Senior, A., C. E. Grueber, T. Kamiya, M. Lagisz, K. O’dwyer, E. S. A. Santos & S. Nakagawa, 2016. Heterogeneity in ecological and evolutionary meta-analyses: its magnitude and implications. Ecology 97: 3293–3299.
Sinev, A. Y. & N. M. Korovchinsky, 2013. Cladocera (Crustacea: Branchiopoda) of Cat Tien National Park, South Vietnam. Journal of Limnology 72: e8.
Soria, M., C. Leigh, T. Datry, L. M. Bini & N. Bonada, 2017. Biodiversity in perennial and intermittent rivers: a meta-analysis. Oikos 126: 1078–1089.
Toussaint, E. F. A., D. Bloom & A. E. Z. Short, 2017. Cretaceous West Gondwana vicariance shaped giant water scavenger beetle biogeography. Journal of Biogeography 44(9): 1952–1965.
Turner, A. M. & S. L. Montgomery, 2009. Hydroperiod, predators and the distribution of physid snails across the freshwater habitat gradient. Freshwater Biology 54: 1189–1201.
Upchurch, P., 2008. Gondwanan break-up: legacies of a lost world? Trends in Ecology and Evolution 23: 229–236.
Vellend, M., D. S. K. Srivastava, M. Anderson, C. D. Brown, J. E. Jankowski, E. J. Kleynhans, N. J. B. Kraft, A. D. Letaw, A. A. M. Macdonald, J. E. Maclean, I. H. Myers-Smith, A. R. Norris & X. Xue, 2014. Assessing the relative importance of neutral stochasticity in ecological communities. Oikos 123: 1420–1430.
Verhoeven, J. T. A., 1980. The ecology of Ruppia-dominated communities in Western Europe II. Synecological Classification, Structure and Dynamics of the Macroflora and Macrofauna Communities. Aquatic Botany 6: 1–85.
Viayeh, M. R. & M. Špolja, 2012. Structure of rotifer assemblages in shallow waterbodies of semi-arid northwest Iran differing in salinity and vegetation cover. Hydrobiologia 686: 73–89.
Viechtbauer, W., 2010. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 36: 1–48.
Vinson, M. R. & C. P. Hawkins, 1998. Biodiversity of stream insects: variation at local, basin, and regional scales. Annual Review of Entomology 43: 271–293.
Wellborn, G. A., D. K. Skelly & E. E. Werner, 1996. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology, Evolution and Systematics 27: 337–363.
Widdicombe, S. & M. C. Austen, 2001. The interaction between physical disturbance and organic enrichment: An important element in structuring benthic communities. Limnology and Oceanography 46: 1720–1733.
Williams, D. D., 1977. Movements of benthos during the recolonization of temporary streams. Oikos 29: 306–312.
Acknowledgements
AFQ thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil (CAPES) – Finance Code 001 and Fundação de Amparo à Pesquisa do Estado de Mato Grosso – (FAPEMAT) (procs. 88887.190907/2018) for providing a student fellowship. ASM and LMB received research fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq procs. 307587/2017-7 and 308974/2020-4, respectively). This work was also developed in the context of the National Institutes for Science and Technology (INCT) in Ecology, Evolution, and Biodiversity Conservation, supported by MCTIC/CNPq (proc. 465610/2014-5) and Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG).
Author information
Authors and Affiliations
Contributions
ACFQ and ASM conceived the ideas for this manuscript. MAP and ACFQ searched the literature and gathered the data. ACFQ conducted the analyses with the help of MAP and LMB. ACFQ wrote the first draft of the manuscript, and all authors contributed to it. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors consent to the publication of this study.
Additional information
Handling editor: Eric R. Larson
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faustino de Queiroz, A.C., Anton-Pardo, M., Bini, L.M. et al. Invertebrate beta diversity in permanent and temporary lentic water bodies: a meta-analytic assessment. Hydrobiologia 849, 1273–1285 (2022). https://doi.org/10.1007/s10750-021-04788-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04788-3