Abstract
Stream macroinvertebrates, as non-target organisms, may face with either a single or a complex stressor during a restoration treatment. We quantified the structural, phylogenetic, and functional responses to both single (water retention) and complex (water retention and grazing) stressors and analysed how the ecological quality changes, in order to reveal the potential unwanted consequences in a soda pan restoration. Species composition has been restructured and taxonomic diversity decreased significantly as a response to the complex stressor, while they did not change under the single stress. Neither the single nor the complex stressor influenced the phylogenetic diversity. Functional diversity showed mainly no or reversible changes. Changes in diversity metrics induced changes in Ecological Quality Ratios, but the ecological state decreased only under the complex stressor. Our results suggest that different facets of alpha diversity (taxonomic, functional and phylogenetic) are complementary and provide different information about community assembly against different stresses. We can support the opinion that taxon, phylogenetic and functional diversity are proper metrics in their own way to assess the impact of stress, but it is necessary to apply them together. Ecological knowledge of this paper could be considered throughout future conservation planning and enhance restoration success in aquatic environments.
Similar content being viewed by others
Introduction
Due to their unique nature and their drastically decreased number, Pannonian salt marshes and soda pans are among the most vulnerable and threatened aquatic habitats in Europe (Boros et al., 2014). These ecosystems are considered as wetlands with international importance under the Ramsar Convention and they are listed in Annex I of the EU Habitats Directive 92/43/EEC (European Commission, 1992). The loss and ecological degradation of such habitats make their restoration inevitable in many cases; yet the management of soda pans and adjacent wetlands needs specialized approaches and criteria. Therefore, the number of implemented restoration projects for these habitats is limited compared to the comprehensive biological knowledge about them (Boros et al., 2014).
In general, soda-pans-related conservation practices primarily target to provide stopover sites for cross-continental migratory birds or breeding or even wintering sites for the local species and to achieve good ecological status by sustaining their natural hydrological cycles (Boros et al., 2013; Stenger-Kovács et al., 2014; Lengyel et al., 2016). Sufficient water supply and suitable water level that maintains the hydrological regime of a pan are generally provided from a surface freshwater stream, which runs along or near the pan. Simultaneously, the area of the soda pans and their adjacent region must be maintained by extensive livestock grazing, which helps to restore the salinity of the soil and generates a mosaic-like vegetation pattern proved to be crucial for waterfowl, shorebirds and waders (Adler et al., 2001; Reeves & Champion, 2004). Besides the primary aim of the restoration, namely, ensuring the appropriate water level or even seasonal dry periods to fulfil the basic requirements of the target group (mostly birds) of the restoration, other points of view and approaches should be taken into consideration to achieve structurally and functionally complex aquatic ecosystems.
Whether a restoration treatment is worth the effort depends greatly on the overall (expected, long term) success of the restoration, including the unwanted symptoms that non-target biota and adjacent habitats have undergone (hereafter ‘side effects’). Side effects do not occur exclusively and strictly within the restored area and habitat, but they may also severely affect the functionally related adjacent habitats not included in the restoration area, but subjected to direct interventions altering some of their characteristics. In the cases of freshwater marsh or pond restoration projects, a good example could be the modification of natural streams in order to provide water supply. The supply streams, regardless of their natural or artificial origin, represent a high ecological value as they contribute to the regional species pool and maintain local biodiversity in an area strongly affected by climate change and the increasing water shortage (Boros et al., 2013). Macroinvertebrates inhabiting these supply streams can be non-target organisms that might be affected by the side effects. Numerous assessment-based studies have reported on the fulfilment of the main aim and reactions of the target group, but side effects of a soda pan-related restoration have barely been noticed and evaluated. This is a serious shortcoming since the realization value of a restoration cannot be assessed without considering the side effects. Soda pan is like a seasonally inundated wetland that is flooded by a supply stream. Water of the soda pan and supply stream is directly, but seasonally related; therefore, supply stream becomes the organic part of the soda pan. During the dry period of the soda pan, the supply stream can provide a shelter for the macroinvertebrates inhabiting the soda pan in the wet period; therefore, it acts as a refugium for them. Aquatic macroinvertebrates are vitally important food for waterfowl that are the target group of restoration treatment (Horváth et al., 2012). The small spatial distance between the soda pan and the supply stream is easily bridgeable by dispersal flight (Boda & Csabai, 2013; Csabai et al., 2012). Tracking supply streams during water retention projects would benefit future efforts to protect and manage stream ecosystems affected by restoration.
Macroinvertebrates in surface freshwater streams (as non-target organisms) may face with either single or combined stressors as a result of the restoration treatment, and the effects of these stressors can be stronger and more manifold for them than what the target group faces. In our case study, treatments caused single stress on one section (water retention along unchanged stream section) and complex stress (water retention and grazing along modified stream section) at another section of a stream (see details in Material and method). Macroinvertebrates in lotic systems can regenerate relatively fast, and the fauna is resettling to its pre-management state within weeks or at most a year after a short-term single disturbances (Whitaker et al., 1979, Yount & Niemi, 1990, Mackay, 1992, Zawal et al., 2016). Long-term effects of two or more stressors on the macroinvertebrate community are difficult to predict because the combination of stressors result in various interactions (Bækkelie et al., 2017; Calapez et al., 2017). Responses to a single or a complex stress can differ in species or higher taxonomic levels and can drive structural or functional changes in the community.
Besides the well-known structural metrics widely used in characterization of aquatic macroinvertebrate communities and in description of their spatiotemporal dynamics including how they respond to disturbances (Bunn & Arthington, 2002; Poff & Zimmerman, 2010; Carlisle et al., 2011), the functional (trait-based) approaches are steadily gaining ground in community ecology providing opportunities for deeper understanding of species responses and ecosystem functioning (McGill et al., 2006; Schmera et al., 2017). In all probability, major changes in functional composition affect ecosystem processes, whereas a change in species diversity may not elicit ecosystem response at all because of the functional redundancy among species. Hence, it is crucial to measure functional diversity to understand the responses of the communities to environmental influences, since functional redundancy can modulate the extent to which any change in the species composition will affect the ecosystem functioning. Diversity of various traits of aquatic macroinvertebrates in a community determines how it can respond to disturbances and how fast it can adapt to the changes in the environment due to a constant single or a complex stressor.
In the present study, we quantified the structural and functional changes in the macroinvertebrate communities of a water supply stream and simultaneously tested how the taxonomic, phylogenetic and functional diversity metrics respond to the presence of a single and a complex stressor, as side effects in a soda pan habitat restoration. We hypothesized that both single (water retention along unchanged stream section) and complex (water retention and grazing along modified stream section) stressors affect taxonomic diversity stronger than functional diversity and the magnitude of this effect is correlated with the complexity of the stressors.
Materials and methods
Study area
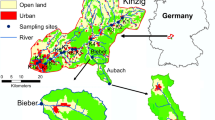
The investigated soda pan (salt marsh) is located near Balmazújváros, in the Hungarian Great Plain, in the middle of Hajdú-Bihar county, (47° 39′ 08″ N, 21° 12′ 56″ E) (Fig. 1). Before the 1910s, in its natural state the soda pan was a soda grassland with periodic inundation from rainwater but without runoff possibilities. Thanks to the lack of runoff, the specific hydrological cycle of the soda pan was maintained by the precipitation, groundwater and evaporation hand in hand. After the World Wars, the deepest part of the soda pan was intersected by a drainage channel, called Magdolna-ér, for the purpose to drain the rainwater from the area, which ultimately doomed the pan to destruction. The Magdolna-ér was only fed by precipitation, collecting the rainfall and draining the inland water from the surrounding area. Since water retention opportunity was not installed, the hydrological cycle changed significantly, and the soda pan became degraded. At the same time, livestock farming and grazing were eliminated from the area. The soda pan was designated as protected only in the early 1990s, and then the entire area was integrated into the NATURA 2000 network. In 2012, two hectares of soda pan bed was restored with the help and financial funding of a LIFE + project (Sodic lake restoration in the Hortobágy). To provide sufficient water supply and maintain the ecologically suitable hydrological regime of the pan, a restoration treatment (including streambed modification and installation of sluices for water retention purposes) was performed only along the section of Magdolna-ér (hereafter supply stream) that runs across the soda pan.
Within the framework of the treatment, the modified section of the Magdolna-ér entirely lost its channel nature. At first, all the aquatic macrophyte stands and the accumulated sediment in 10 cm depth were removed using an excavator. Then a 5–10 m wide and flat streambed were created with a bank slope of 1:5. After the streambed modification, Magdolna-ér has a maximum depth of 60–80 cm and a 15–20 m wide water surface. Finally, a sluice was installed to the lower edge of the pan (eastern sluice), and another one was installed far before the upper edge of the pan (upper sluice). Weed cutting, dredging, streambed modification and sluices installation were performed to facilitate water retention. Streambed modification was not performed on the stream section between the eastern edge of soda pan and the eastern sluice (i.e. unchanged stream section). At present, there are two ways to provide water supply in the soda pan: the natural way is from precipitation and the artificial way is flooding from Magdolna-ér controlled by conservationists. The upper sluice was open from October to March, while the lower sluice was locked all year round. Retained amount of water is 256.800 m3/year. After the streambed restoration, 1.5 Livestock unit/ha have started to graze continuously on the soda pan (including Magdolna-ér). The area was utilized by ecologically sustainable high-level grazing and the main goal of the soda-pan restoration was achieved. However, due to treatments a single stress appeared on the site S (water retention along unchanged stream section), and a complex stress (water retention and grazing along modified stream section) appeared on the site C of the supply stream.
Study design
Restoration work has created two types of impact on the Magdolna-ér. In the section located outside (i.e. upstream) of the newly restored soda pan, the streambed is subjected to a single stressor (water retention) without streambed modification and grazing (sampling site at this section mentioned hereafter as site S). The section located in the pan is simultaneously subjected to multiple stressors (water retention, grazing, and streambed modification) having a complex impact on stream (hereafter site C). Three samplings were carried out in the years of 2011, 2013 and 2014, while samples were not taken in the year of 2012 when reconstruction works were performed. Thus, the pre-treatment status of the stream in 2011 was considered as a baseline and named as C0 and S0. The status recorded in the two consecutive years after the treatment were coded as C1, C2 and S1, S2, respectively. To help the interpretation of the results, hereinafter, we are referring to the combination of treatment and years in the way as shown in Fig. 1. The number of sites and numbers of spatial replicates in each site were relatively low (3–3 in sites S and C, respectively); however, it is justified by the quite small area of the restoration: involving more sites could have increased redundancy. Although only one year was available for baseline sampling, it is counterbalanced by the three-seasonal sampling design for both the baseline and ‘after treatment’ years.
Macroinvertebrate sampling
Three sampling points in both of site C and S of the Magdolna-ér were assigned to ensure spatial replications. In order to have temporal replications and to represent the seasonal variation in the macroinvertebrate community the stream was sampled three times (spring, summer and autumn) in each year at both sites. Altogether, 54 semi-quantitative samples were conducted during the sampling campaign (2011, 2013, 2014). AQEM multi-habitat sampling method was applied with “kick and sweep” technique using a hand net with 500 µm mesh size (AQEM Consortium, 2002; Hering et al., 2003). The samples were performed by one person throughout the sampling years. The samples preserved with 70% ethanol were stored in plastic containers and transferred to the laboratory, where the sorting process was performed later picking all individuals from the samples. Individuals of macroinvertebrates from 12 taxonomic groups (Gastropoda, Bivalvia, Oligochaeta, Hirudinea, Crustacea, Ephemeroptera, Odonata, Heteroptera, Coleoptera, Megaloptera, Trichoptera, and Diptera) were identified under stereomicroscope to the lowest possible taxonomic level, mostly to species-level. The sorted and identified individuals were preserved in 70% ethanol.
Data analysis
Scatterplot derived from Non-metric Multidimensional Scaling (NMDS, Bray–Curtis dissimilarity, abundance data) and Permutational multivariate analysis of variance (PERMANOVA, Anderson et al., 2001) were used to analyse the macroinvertebrate community composition through time. Before analyses, raw data were pooled yearly and transformed into relative abundances; thus, one year was represented by data triplets (3 sampling points as spatial replication, each sampled by 3 times per year). Diversity in both sites and years was compared by Rényi’s diversity profiles (Kindt et al., 2006; Borics et al., 2021). Rényi diversity ordering (1961) is using a scale parameter (α) generalizing the Shannon’s entropy. When the diversity profile of an assemblage is above or equal to the diversity profile of another assemblage over the entire range of the scale parameter then the first assemblage is more diverse than the second one. However, the curves of two diversity profiles may intersect, but the ecological meaning which refers to the correlated assemblages depends on the position of the intersection. When the value of the scale parameter is 0, then the value of Rényi diversity is the logarithm of the number of species. If the value of the scale parameter is 1, then the Rényi diversity is identical with the Shannon index of diversity. When the value of the scale parameter is 2, the Rényi diversity is related to the quadratic (Simpson) diversity. Finally, when the value of the scale parameter is large (approaches positive infinity) the Rényi diversity is equivalent to Berger-Parker dominance index (Tóthmérész, 1995). Macroinvertebrate community composition (NMDS, PERMANOVA) and diversity (Rényi profiles) were analysed and calculated by using the package vegan (ver 2.5) in R environment (Oksanen et al., 2013).
Two-way ANOVA analyses with Tukey post hoc tests conducted in STATISTICA (version 10) software (StatSoft Inc.,Tulsa, OK, USA) were used to compare values of structural and functional metrics between sites and years. Four structural metrics were calculated for community level (Number of individuals and species, Shannon diversity, Evenness) and two structural metrics were calculated for higher taxa level (Number of individuals and species). Altogether, 65 functional traits of macroinvertebrate taxa were acquired from www.freshwaterecology.info (Schmidt-Kloiber & Hering, 2015). Functional trait data were downloaded for species and also for genera. We only selected functional traits if their coverage in the database was higher than 10%, thus 30 species-level, and 24 genus-level functional traits remained for analyses (ESM Table 1). Community-weighted mean traits (CWM) were calculated from the relative abundance and the selected trait data by CANOCO 5 (Šmilauer & Lepš, 2014) to generate raw trait matrix. Functional Shannon diversity and functional Evenness values were calculated by PAST (ver 3, Hammer et al., 2001) based on raw trait matrix.
To describe the phylogenetic diversity related to site and years, we calculated average taxonomic distinctness (AvTD, Δ+) based on yearly pooled data with the help of PRIMER v6 software (Clarke & Gorley, 2006). The results were displayed in funnel plots, where the mean Δ+ and 95% probability limits were calculated based on 999 random selections for each number of species from the total species list.
The Hungarian multimetric index (HMMI) value, expressed as Ecological Quality Ratio (EQR) was used to assess whether the potential changes in species composition, structural and functional features can cause ecological quality changes. The HMMI_sl metric was used for analysis, which is a specific multimetric index that fulfils the intercalibration procedure for assessing ecological quality of small lowland streams. The HMMI_sl contains four metrics: EPTCOB (Ephemeroptera + Plecoptera + Trichoptera + Coleoptera + Odonata + Bivalvia total taxon number), SH (Shannon diversity metric), ASPT (average score per taxon) and LR% (limno- to rheophil zonation preference percentage). Thus, the index/ecological state of a lowland stream was calculated as follows.
Results
During the sampling campaign, 54 samples yielded 11,548 aquatic macroinvertebrate specimens belonging to 245 taxa, from which 176 were identified to species-level. The number of taxa within the 12 higher level group of organisms were as follows: Bivalvia—4; Gastropoda—29; Oligochaeta—1; Hirudinea—10; Crustacea—5; Ephemeroptera—4; Odonata—21; Heteroptera—25; Coleoptera—114; Megaloptera—1; Trichoptera—7; Diptera—24.
The cumulative number of taxa (three years and all spatial and seasonal replicates were pooled) in site S was higher (192) than that in site C (160). More taxa were found in S1 and S2 than S0 (129 and 128 versus 112), while C1 and C2 had a lower number of taxa than C0 (89, 81, 112, respectively). 68 taxa were found exclusively in site S, while 36 in site C (Fig. 2). No differences were revealed between years by Two-way ANOVA based on number of individuals and number of species (number of species: P = 0.0664, number of individuals: P = 0.071, ESM Table 2) in each site.
NMDS ordination revealed that the sites S and C were very similar in the year before the treatment (C0, S0, 2011). One year after the reconstruction works (C1, S1, 2013), composition of S changed only slightly ,while C shifted to a highly different status with a very high variance. Two years after the treatment (C2, S2, 2014) the variance detected in C samples decreased but its composition became more different from its zero status and highly different from S2 too (Fig. 3). The effect of grouping (sites × years) was significant based on PERMANOVA (SSwithin = 2.831, SStotal = 4.877, F = 1.734, P = 0.0008), but the pairwise comparisons did not show clear separation due to the small number of cases.
Scatterplot of the Non-metric multidimensional scaling (NMDS) based on the abundance data related to sites and years revealed smaller changes in site S trough years than in site C. Convex hulls indicated the three spatial replicates from a given year at each site. C = complex stressor site, S = single stressor site, numbers refer to as follows: 0 = 2012 (before the treatment), 1 = 2013 (one year after treatment) and 2 = 2014 (two years after treatment). Stress = 0.1137
Rényi’s diversity profiles show that the diversity of the macroinvertebrate community changed much less after the treatment in site S than in site C. Regarding site S, the changes cannot be interpreted as significant and clear because the diversity profiles are crossing each other meaning that the abundances of dominant and rare species changed in different directions. In contrast, the diversity of the community in site C decreased clearly and significantly compared to the zero status (Fig. 4). The two attributes of diversity, such as species number and species inequalities, show the same trends in three years resulting in almost identical values of each index constituting the special cases of Rényi’s entropy (species richness, Shannon, Simpson, Berger-Parker).
Rényi’s diversity profiles of communities at sites S and C. Values at alpha on the y-axis refer to as follows: 0 = corresponds to species richness, 1 = represents the Shannon diversity and 2, 3 and 4 = higher values corresponding to greater evenness. C0 represents an unambiguously more diverse community than C1 and C2 because the profiles do not cross each other. In S site, the changes are not significant because the diversity profiles are crossing each other
Based on the number of individuals, all but one higher taxa had no significant temporal differences at both sites: in site S the number of Hirudinea specimens, while in site C the number of Coleoptera specimens was lower year by year (Two-way ANOVA, Fig. 5A, B). More changes were detected in the numbers of species: 4 out of 12 higher taxa had significant temporal differences at both sites. Year by year, the species number of Crustacea, Diptera and Odonata significantly increased, and that of Hirudinea significantly decreased in site S, while the species number of Crustacea and Diptera significantly increased and that of Hirudinea and Coleoptera significantly decreased in site C (Two-way ANOVA, Fig. 5C, D, see ESM Table 3).
Graphical summary of year-to-year relative changes based on the results of Two-way ANOVA analyses. Small-scale irreversible changes can be seen both in the case of higher taxa and in the diversity of functional traits. A–D Relative changes in number of individuals (A, B) and number of species (C, D) based on higher taxa; E–H relative changes of diversity of functional traits at Species-level (E, F) and Genus-level (G, H). Blue arrows and bars refer to the site S, while red arrows and bars represent the site C. Relative changes can be defined as the difference between C0 and C2 (Two-way ANOVA with post hoc Tukey pairwise tests, statistical significance at the P < 0.05 level). Reversible changes mean significant differences could be detected only between C0 and C1, but not between C0 and C2. Irreversible changes mean that significant differences could be detected between years 0 and 2 too. Higher taxa (orders) are indicated with pictograms. Width of the bars in panels E–H represents the extent of the changes
The year-to-year changes of the functional diversity were different between the sites C and S based on Two-way ANOVA analyses. In site S, 90.0% (at species-level) and 76.9% (at genus-level) of the cases (i.e. the diversity of the given trait) showed no significant changes, while these ratios were much lower in site C (50.0% at species-level and 42.3% at genus-level). Alternately, reversible changes were only observed in few cases (3.3% at species-level and 3.8% at genus-level) in site S, while the ratio of non-lasting changes was remarkably higher in site C (36.7% at species-level, and 42.3% at genus-level). The ratio of significant and irreversible changes was much higher in site C (13.3%) than in site S (6.7%) at species-level, while at genus-level the difference was not remarkable between the sites (19.2% in site S and 15.4% in site C) (Two-way ANOVA, Fig. 5E–H, see ESM Table 4).
Although the C0 Δ + was higher than the mean with a relatively high number of species, the Δ + values among sites and years did not differ significantly. Both Δ + and species number remarkably decreased in C1, the Δ + value was below the mean. In C2 the number of species decreased further, but the Δ + became higher than in C0 and almost significantly (P = 0.076) higher than the mean. In S0 the Δ + is almost equivalent with the mean value with a relatively high number of species. Although the number of species increased in S1, Δ + strikingly decreased and its value was marginally significantly under the mean. In S3 the number of species did not change, while the Δ + slightly increased but remained below the mean (Fig. 6).
Taxonomic distinctness analyses of species composition of sites and years. Either the single or the complex stress does not cause changes in phylogenetic diversity. Dots refer to sites and years (blue S: site affected by the single stressor, red C: site affected by the complex stressor, numbers refer to the years as in Fig. 1). The horizontal line represents the mean TD value for the total set of collected species and the funnel indicates the confidence interval at 95%
Ecological quality ratios indicated good ecological status in site S continuously throughout the entire period (2011–2014). The ecological status also was good in site C before the reconstruction work, but after the treatment the quality status has fallen to the moderate category, even to the lower quarter of this and remained there for the next year too (Fig. 7).
Plots of ecological quality ratio (EQR) scores related to sites and years. Ecological quality class (EQC) has fallen from good to moderate in the site C, but does not change in site S. EQR defines ecological quality classes (EQCs) of surface waters. The EQR is expressed as a numerical value between zero (worse class) and one (best class). Then five quality classes can be created: high (blue background), good (green background), moderate (yellow background), poor (orange background) and bad (red background). Red plots represent the Complex site in different years (C), and blue plots represent the Single site in different years (S). EQC terminology and colour-coding are according to EC (2000)
Discussion
Through a case study, we reveal here how a restoration treatment can affect non-target biota and a non-target habitat spot beside its positive effect on the targeted ones. Generally, the restoration treatment carried out on the supply stream generated single and complex stress on macroinvertebrates in given sections of the supply stream. The effects of a single and a complex stressor on macroinvertebrates is well studied generally (e.g. Hering et al., 2015; Graeber et al., 2017, de Vries et al., 2019), but as far as we know, it has not been studied in small-scale restoration projects so far. Here we reveal for the first time that they can cause changes in macroinvertebrate community composition and trait diversity in different ways.
Our results are in line with previous ones, as stress (either complex or single) induces profound (e.g. Horsák et al., 2009; Wyżga et al., 2012) or moderate (e.g. Turunen et al., 2016) structural changes in the composition of the macroinvertebrate communities. Modification of the streambed by dredging, water retention, livestock grazing and the interactions between them completely changed the stream habitat, which is no longer coherent with the ecological demand of the members of the original community. Accordingly, the structural changes in the macroinvertebrate assemblages was manifested through the disappearance of several species and the formation of a new taxonomic structure, as it was found in previous studies as well (e.g. Perring et al., 2013; Bozóki et al., 2018).
Single stress has no effect on structural Shannon diversity, but the changed species composition in complex sites implies a decrease in taxonomic diversity. This decline is in accord with recent studies indicating the negative effect of the complex stress on macroinvertebrate diversity (Rasmussen et al., 2013; Turunen et al., 2016; Graeber et al., 2017; Beermann et al., 2018).
Although structural changes in species composition and taxonomic diversity are substantial, the present results have demonstrated that community effects are generally more detectable on higher levels of taxonomic resolution such as family or order level (Mueller et al., 2011). Actually, in the level of taxonomic orders, the number of individuals show low (8%) responsiveness to water retention in site S, but the number of species gives higher irreversible response (25%). However, stress-induced changes in species composition (even smaller or higher) in both sites do not cause changes in phylogenetic diversity. It means that the evolutionary relationships among species both in initial and new communities are almost the same regardless of the condition of the habitats. Phylogenetic diversity of the community ‘per se’ does not give insight to the changes of evolutionary information, that may explain why the role of phylogenetic diversity in conservation planning is contradictory (Winter et al., 2013).
We could not detect any influences of neither the single nor the complex stressor on most of the major taxonomic groups (Gastropoda, Bivalvia, Oligochaeta, Ephemeroptera, Heteroptera, Megaloptera, Trichoptera). However, the affected Crustacea, Hirudinea, Diptera, Coleoptera and Odonata assemblages reflected the stressors differently. Although understanding the underlying processes behind the taxon-specific responses could be important in assessment of the effects of restoration treatments, the detailed explanation is far beyond the goals of this study, and we focus on the general patterns of changes.
Functional diversity (characterized by the Shannon diversity of a given trait) has changed year by year at both sites, but only less than 20% of functional traits indicated irreversible changes. The effect of the altered flow regime on flow-related traits is well documented (Statzner et al., 1988; Allan & Castillo, 2007); however, water current also affects the sedimentation, food availability, temperature and salinity characteristics, and chemical features of water which may explain changes in several traits (feeding type, current velocity preference, larval development, longitudinal distribution, salinity, temperature range; see ESM Table 3). In addition, negative effects of streambed modification (Horsák et al., 2009; Wyżga et al., 2012; Turunen et al., 2016) and order-specific effects of grazing (Silver & Vamosi, 2012) on macroinvertebrates have been proven previously. These stressors have strong effects on macroinvertebrates, and synergistic effects of them also can cause differences in functional diversity (Martínez et al., 2013; Linares et al., 2019). Functional diversity changes are also more detectable in genus-level than species-level, but it may also be influenced by the different coverage of genus-level and species-level traits in databases (see Material and method and ESM Table 1).
Changes in the structural, functional and phylogenetic diversity as a response to stressors can induce changes in the ecological state of the stream. Although structural changes can influence ecosystem functioning through functional redundancy indirectly, after all, functional diversity is what primary facet of diversity that provides the link between ecosystem functioning (Tilman et al., 1997; Petchey & Gaston, 2006; Schmera et al., 2017). A variety of linkages between functional diversity and ecosystem functioning is possible (Cao et al., 2018), but if the habitat is in good status—that means it is just slightly worse than the reference status—based on an ecological quality element, then the other (biological, hydromorphological, physico-chemical, and chemical) quality elements should ensure ecosystem functioning (European Commission, 2003; Van de Bund & Solimini, 2007). This theoretical deduction might be valid for site S, as the structural and functional changes induce changes in the values of Ecological Quality Ratios (EQRs), but the Ecological Quality Class (EQC, determined by EQRs) has not changed. This means that the ~ 20% irreversible changes in functional diversity caused by species composition changes have not reached the threshold among the classes, and it cannot be interpreted as decreases in ecosystem functioning. In contrast, a smaller change in functional diversity (~ 15.4%) resulted in that EQC has fallen from good to moderate in the site C. The only explanation for this apparent contradiction can be that the trait redundancy is different between sites affected by the single or the complex stressor. In other words, loss of species in case of the single stressor may be balanced by other species exhibiting similar traits and contributing similarly to the functional diversity of the community, while in case of the complex stressor, new species with new traits inhabit the habitat offering new circumstances.
Our results are not completely in line with previous suggestions that stressors have less impact on functional and phylogenetic diversity than on taxonomic diversity (Vaughn, 2010; Feld et al., 2014; Podani et al. 2018). However, our findings suggest that aquatic macroinvertebrates contribute to ecosystem functioning via their functional features rather than taxonomic characteristics (Lavorel et al., 1997; Schmera et al., 2017). The different response in functional and phylogenetic level may be due to that the functional and phylogenetic diversity are related to different environmental drivers. It suggesting that different facets of alpha diversity are complementary (e.g. Moreno et al., 2009) and provide different information about community assembly against different stresses or sets of stressors (e.g. Münkemüller et al., 2012). Based on our results, we can support the opinion that taxon diversity, functional diversity and phylogenetic diversity are proper metrics in their own way to assess the impact of stress on macroinvertebrates (Saito et al., 2015; England & Wilkes, 2017), but to see the whole, often explicitly complex picture, to explore and understand every detail, it is necessary to apply them together.
Conclusion
In our case study, the macroinvertebrates inhabiting the supply stream were not the target group, but they suffered from the side effects of the human-induced restoration treatment. This study attracts further attention to the importance of the side effects and unwanted consequences of conservational treatments in aquatic ecosystems. Interaction of the complex stressor jeopardizes biodiversity, function, and processes, thus ecological knowledge of this paper could be considered throughout conservation planning and enhance restoration success in the future (Côté et al., 2016). Since supply streams of a restoration may represent high ecological value contributing to the regional species pool and maintaining local biodiversity in several ways, by providing a more diverse habitat structure and shelter in water shortage periods, we recommend paying attention to the side effects and essentially incorporate tracking ecological changes of supply streams to the biological monitoring during restoration projects to achieve a better understanding of the overall functioning of the system as a whole.
References
Adler, P., D. Raff & W. Lauenroth, 2001. The effect of grazing on the spatial heterogeneity of vegetation. Oecologia 128: 465–479.
Allan, J. D. & M. M. Castillo, 2007. Stream Ecology: Structure and Function of Running Waters. Springer, New York.
Anderson, M. J., 2001. A new method for non-parametric multivariate analysis of variance. Austral ecology 26: 32–46.
Bækkelie, K. A. E., S. C. Schneider, C. H. Hagman & Z. Petrin, 2017. Effects of flow events and nutrient addition on stream periphyton and macroinvertebrates: an experimental study using flumes. Knowledge & Management of Aquatic Ecosystems 418: 47.
Beermann, A. J., V. Elbrecht, S. Karnatz, L. Ma, C. D. Matthaei, J. J. Piggott & F. Leese, 2018. Multiple-stressor effects on stream macroinvertebrate communities: A mesocosm experiment manipulating salinity, fine sediment and flow velocity. Science of the Total Environment 610: 961–971.
Boda, P. & Z. Csabai, 2013. When do beetles and bugs fly? A unified scheme for describing seasonal flight behaviour of highly dispersing primary aquatic insects. Hydrobiologia 703: 133–147.
Borics, G., A. Abonyi, N. Salmaso & R. Ptacnik, 2021. Freshwater phytoplankton diversity: models, drivers and implications for ecosystem properties. Hydrobiologia 848: 53–75. https://doi.org/10.1007/s10750-020-04332-9
Boros, E., Z. Ecsedi, J. Oláh, S. Regina & J. Dunn, 2013. Ecology and management of soda pans in the Carpathian Basin. Hortobágy Environmental Association, Balmazújváros.
Boros, E., Z. Horváth, G. Wolfram & L. Vörös, 2014. Salinity and ionic composition of the shallow astatic soda pans in the Carpathian Basin. Annales de Limnologie - International Journal of Limnology 50: 59–69.
Bozóki, T., E. Á. Krasznai-Kun, A. Csercsa, G. Várbíró & P. Boda, 2018. Temporal and spatial dynamics in aquatic macroinvertebrate communities along a small urban stream. Environmental Earth Sciences 77: 559.
Bunn, S. E. & A. H. Arthington, 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30: 492–507.
Calapez, A. R., P. Branco, J. M. Santos, T. Ferreira, T. Hein, A. G. Brito & M. J. Feio, 2017. Macroinvertebrate short-term responses to flow variation and oxygen depletion: a mesocosm approach. Science of the Total Environment 599: 1202–1212.
Cao, X., L. Chai, D. Jiang, J. Wang, Y. Liu & Y. Huang, 2018. Loss of biodiversity alters ecosystem function in freshwater streams: potential evidence from benthic macroinvertebrates. Ecosphere 9: e02445.
Carlisle, D. M., D. M. Wolock & M. R. Meador, 2011. Alteration of streamflow magnitudes and potential ecological consequences: a multiregional assessment. Frontiers in Ecology and the Environment 9: 264–270.
Clarke, K. & R. Gorley, 2006. “PRIMER v6.” user manual/tutorial, Plymouth, UK.
Commission, European, 1992. Habitats Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Official Journal of the European Union 206: 7–50.
Côté, I. M., E. S. Darling & C. J. Brown, 2016. Interactions among ecosystem stressors and their importance in conservation. Proceedings of the Royal Society B: Biological Sciences 283: 20152592.
Csabai, Z., Z. Kálmán, I. Szivák & P. Boda, 2012. Diel flight behaviour and dispersal patterns of aquatic Coleoptera and Heteroptera species with special emphasis on the importance of seasons. Naturwissenschaften 99: 751–765.
de Vries, J., M. H. Kraak, R. C. Verdonschot & P. F. Verdonschot, 2019. Quantifying cumulative stress acting on macroinvertebrate assemblages in lowland streams. Science of the Total Environment 694: 133630.
England, J. & M. A. Wilkes, 2018. Does river restoration work? Taxonomic and functional trajectories at two restoration schemes. Science of the Total Environment 618: 961–970.
European Commission 2003. River and lakes – Typology, reference conditions and classification systems. Common Implementation Strategy for the Water Framework Directive 2000/60/EC, Guidance document n.o 10, Produced by working group 2.3. Official publications of the European Community, Luxembourg.
Feld, C. K., F. de Bello & S. Dolédec, 2014. Biodiversity of traits and species both show weak responses to hydromorphological alteration in lowland river macroinvertebrates. Freshwater Biology 59: 233–248.
Graeber, D., T. M. Jensen, J. J. Rasmussen, T. Riis, P. Wiberg-Larsen & A. Baattrup-Pedersen, 2017. Multiple stress response of lowland stream benthic macroinvertebrates depends on habitat type. Science of the Total Environment 599: 1517–1523.
Hammer, Ø., D. A. Harper & P. D. Ryan, 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 9.
Hering, D., A. Buffagni, O. Moog, L. Sandin, M. Sommerhäuser, I. Stubauer, C. Feld, R. Johnson, P. Pinto, N. Skoulikidis, P. Verdonschot & S. Zahrádková, 2003. The development of a system to assess the ecological quality of streams based on macroinvertebrates – Design of the sampling programme within the AQEM project. International Review of Hydrobiology 88: 345–361.
Hering, D., L. Carvalho, C. Argillier, M. Beklioglu, A. Borja, A. C. Cardoso, H. Duel, T. Ferreirea, L. Globevnik, J. Hanganu, S. Hellsten, E. Jeppesen, V. Kodeš, A. Lyche Solheim, T. Nõges, S. Ormerod, Y. Panagopoulos, S. Schmutz, M. Venohr & S. Birk, 2015. Managing aquatic ecosystems and water resources under multiple stress – An introduction to the MARS project. Science of the Total Environment 503: 10–21.
Horsák, M., J. Bojková, S. Zahrádková, M. Omesová & J. Helešic, 2009. Impact of reservoirs and channelization on lowland river macroinvertebrates: A case study from Central Europe. Limnologica 39: 140–151.
Horváth, Zs, M. Ferenczi, A. Móra, C. F. Vad, A. Ambrus, L. Forró, G. Szövényi & S. Andrikovics, 2012. Invertebrate food sources for waterbirds provided by the reconstructed wetland of Nyirkai-Hany, northwestern Hungary. Hydrobiologia 697: 59–7.
Kindt, R., P. Van Damme & A. J. Simons, 2006. Tree diversity in western Kenya: using profiles to characterise richness and evenness. Forest Diversity and Management Springer, Dordrecht.
Lavorel, S., S. McIntyre, J. Landsberg & T. D. A. Forbes, 1997. Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends in Ecology & Evolution 12: 474–478.
Lengyel, E., J. Padisák, É. Hajnal, B. Szabó, A. Pellinger & C. Stenger-Kovács, 2016. Application of benthic diatoms to assess efficiency of conservation management: a case study on the example of three reconstructed soda pans, Hungary. Hydrobiologia 777: 95–110.
Linares, M. S., W. Assis, R. R. de Castro Solar, R. P. Leitão, R. M. Hughes & M. Callisto, 2019. Small hydropower dam alters the taxonomic composition of benthic macroinvertebrate assemblages in a neotropical river. River Research and Applications 35: 725–735.
Mackay, R. J., 1992. Colonization by lotic macroinvertebrates: a review of processes and patterns. Canadian Journal of Fisheries and Aquatic Sciences 49: 617–628.
Martínez, A., A. Larranaga, A. Basaguren, J. Perez, C. Mendoza-Lera & J. Pozo, 2013. Stream regulation by small dams affects benthic macroinvertebrate communities: from structural changes to functional implications. Hydrobiologia 711: 31–42.
McGill, B. J., B. J. Enquist, E. Weiher & M. Westoby, 2006. Rebuilding community ecology from functional traits. Trends in Ecology & Evolution 21: 178–185.
Moreno, C. E., G. Castillo-Campos & J. R. Verdú, 2009. Taxonomic diversity as complementary information to assess plant species diversity in secondary vegetation and primary tropical deciduous forest. Journal of Vegetation Science 20: 935–943.
Mueller, M., J. Pander & J. Geist, 2011. The effects of weirs on structural stream habitat and biological communities. Journal of Applied Ecology 48: 1450–1461.
Münkemüller, T., S. Lavergne, B. Bzeznik, S. Dray, T. Jombart, K. Schiffers & W. Thuiller, 2012. How to measure and test phylogenetic signal. Methods in Ecology and Evolution 3: 743–756.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’hara, G. L. Simpson, P. Solymos, M. H. N. Stevens, E. Szoecs, H. Wagmer, 2013. Package ‘vegan’. Community ecology package, version, 2: 1–295
Perring, M. P., R. J. Standish & R. J. Hobbs, 2013. Incorporating novelty and novel ecosystems into restoration planning and practice in the 21st century. Ecological Processes 2: 18.
Petchey, O. L. & K. J. Gaston, 2006. Functional diversity: back to basics and looking forward. Ecology Letters 9: 741–758.
Podani, J., S. Pavoine & C. Ricotta, 2018. A generalized framework for analysing taxonomic, phylogenetic, and functional community structure based on presence–absence data. Mathematics 6: 250.
Poff, N. L. & J. K. Zimmerman, 2010. Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshwater Biology 55: 194–205.
Rasmussen, J. J., U. S. McKnight, M. C. Loinaz, N. I. Thomsen, M. E. Olsson, P. L. Bjerg, P. J. Binning & B. Kronvang, 2013. A catchment scale evaluation of multiple stressor effects in headwater streams. Science of the Total Environment 442: 420–43.
Reeves, P. N. & P. D. Champion, 2004. Effects of livestock grazing on wetlands: literature review. Environment Waikato Regional Council.
Rényi, A. 1961. On measures of entropy and information. In Proceedings of the Fourth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1: Contributions to the Theory of Statistics. The Regents of the University of California.
Saito, V. S., T. Siqueira & A. A. Fonseca-Gessner, 2015. Should phylogenetic and functional diversity metrics compose macroinvertebrate multimetric indices for stream biomonitoring? Hydrobiologia 745: 167–179.
Schmera, D., J. Heino, J. Podani, T. Erős & S. Dolédec, 2017. Functional diversity: a review of methodology and current knowledge in freshwater macroinvertebrate research. Hydrobiologia 787: 27–44.
Schmidt-Kloiber, A. & D. Hering, 2015: www.freshwaterecology.info - an online tool that unifies, standardises and codifies more than 20,000 European freshwater organisms and their ecological preferences
Silver, C. A. & S. M. Vamosi, 2012. Macroinvertebrate community composition of temporary prairie wetlands: a preliminary test of the effect of rotational grazing. Wetlands 32: 185–197.
Šmilauer, P. & J. Lepš, 2014. Multivariate analysis of ecological data using CANOCO 5. Cambridge University Press, Cambridge.
Statzner, B., J. A. Gore & V. H. Resh, 1988. Hydraulic stream ecology: observed patterns and potential applications. Journal of the North American Benthological Society 7: 307–360.
Stenger-Kovács, C., E. Lengyel, K. Buczkó, F. M. Tóth, L. O. Crossetti, A. Pellinger, Z. Zámbóné Doma & J. Padisák, 2014. Vanishing world: alkaline, saline lakes in Central Europe and their diatom assemblages. Inland Waters 4: 383–396.
Tilman, D., J. Knops, D. Wedin, P. Reich, M. Ritchie & E. Siemann, 1997. The influence of functional diversity and composition on ecosystem processes. Science 277: 1300–1302.
Tóthmérész, B., 1995. Comparison of different methods for diversity ordering. Journal of vegetation Science 6: 283–290.
Turunen, J., T. Muotka, K. M. Vuori, S. M. Karjalainen, J. Rääpysjärvi, T. Sutela & J. Aroviita, 2016. Disentangling the responses of boreal stream assemblages to low stressor levels of diffuse pollution and altered channel morphology. Science of the Total Environment 544: 954–962.
Van de Bund, W. & A. Solimini, 2007. Ecological Quality Ratios for ecological quality assessment in inland and marine waters. Institute for Environment and Sustainability, Ispra.
Vaughn, C. C., 2010. Biodiversity losses and ecosystem function in freshwaters: emerging conclusions and research directions. BioScience 60: 25–35.
Whitaker, G. A., R. H. McCuen & J. Brush, 1979. Channel modification and macroinvertebrate community diversity in small streams 1. JAWRA Journal of the American Water Resources Association 15: 874–879.
Winter, M., V. Devictor & O. Schweiger, 2013. Phylogenetic diversity and nature conservation: where are we? Trends in Ecology & Evolution 28: 199–204.
Wyżga, B., J. Zawiejska, A. Radecki-Pawlik & H. Hajdukiewicz, 2012. Environmental change, hydromorphological reference conditions and the restoration of Polish Carpathian rivers. Earth Surface Processes and Landforms 37: 1213–1226.
Yount, J. D. & G. J. Niemi, 1990. Recovery of lotic communities and ecosystems from disturbance—a narrative review of case studies. Environmental Management 14: 547–569.
Zawal, A., A. Sulikowska-Drozd, E. Stępień, Ł. Jankowiak & A. Szlauer-Łukaszewska, 2016. Regeneration of the molluscan fauna of a small lowland river after dredging. Fundamental and Applied Limnology/Archiv Für Hydrobiologie 187: 281–293.
Acknowledgments
We thank András Csercsa, Ágnes Győri, Judit Horváth, Máté Bolbás, Barbara Palombi, Vivien Viski, Judit Csaba, Erika Mihaliczku, Renáta Tóth, and Zsuzsanna Urbán (former students of University of Debrecen, Hungary) for extensive help during field work and Csaba Deák for identification. The authors thank Zoltán Ecsedi (Hortobágy Environmental Association) for the opportunity to perform this study under the LIFE + project. PB, TB, GV and EKK were financially supported by the GINOP-2.3.2-15-2016-00019 project. ZC and AM were supported by the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the 20765-3/2018/FEKUTSTRAT and TUDFO/47138/2019-ITM “Innovation for sustainable and healthy living and environment” thematic programme of the University of Pécs and by the EU-funded project no. EFOP-3.6.1.-16-2016-00004. PB, VG, ZC and TB were supported by NKFIH OTKA FK-135136. EKK was supported by the TKP2020-IKA-07 project financed under the 2020-4.1.1-TKP2020 Thematic Excellence Programme by the National Research, Development and Innovation Fund of Hungary.
Funding
Open Access funding provided by ELKH Centre for Ecological Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Dani Boix
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boda, P., Bozóki, T., Krasznai-K., E.Á. et al. Restoration-mediated alteration induces substantial structural changes, but negligible shifts in functional and phylogenetic diversity of a non-target community: a case study from a soda pan. Hydrobiologia 848, 857–871 (2021). https://doi.org/10.1007/s10750-020-04494-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04494-6