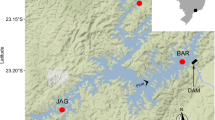
Abstract
We investigated how the riverine network influences taxonomic and functional beta diversity patterns of fish assemblages in the mainstem/headwater (lateral) and upstream/downstream (longitudinal) gradients in a Neotropical river system. We investigated the following questions: which component (turnover or nestedness) explains taxonomic and functional beta diversity in both gradients? Is this component a consistent pattern for different lateral sections of the river basin? Is this component influenced by the spatial extent? Finally, how are taxonomic and functional beta diversity structured by space and environment along the longitudinal gradient? Taxonomic and functional turnover were the main patterns found for the lateral gradient and they were consistent for all lateral sections considered. Taxonomic and functional turnover were also the main patterns for the longitudinal gradient, increasing with the spatial extent increase and being structured by space and spatially structured environments. Our study demonstrates that the dendritic nature of riverine systems constrains species and traits occurrence along lateral and longitudinal gradients in a Neotropical region, generating taxonomic and functional turnover patterns due to the influence of space and spatially structured environments on niche- and dispersal-based processes. These results show that Neotropical riverine systems conservation must go beyond traditional approaches and consider the metacommunity perspective.

Similar content being viewed by others
References
Altermatt, F., 2012. Metacommunity dynamics. In Gibson, D. (ed.), Oxford Bibliographies Online: Ecology. Oxford University Press, New York.
Altermatt, F., 2013. Diversity in riverine metacommunities: a network perspective. Aquatic Ecology 47: 365–377.
Altermatt, F., M. Seymour & N. Martinez, 2013. River network properties shape a-diversity and community similarity patterns of aquatic insect communities across major drainage basins. Journal of Biogeography 40: 2249–2260.
Azevedo, M. C. C., F. G. Araújo, A. G. Cruz-Filho, A. L. M. Pessanha, M. A. Silva & A. P. P. Guedes, 2007. Demersal fishes in a tropical bay in southeastern Brazil: partitioning the spatial, temporal and environmental components of ecological variation. Estuarine, Coastal and Shelf Science 75: 468–480.
Barbalho, M. G. S., A. C. Leal, J. O. R. Nunes, C. G. Moraes & J. C. Peixoto, 2018. Unidades da paisagem da bacia do Rio das Almas, microrregião de Ceres/GO. Planeta Amazônia 10: 153–166.
Baselga, A., 2010. Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography 19: 134–143.
Baselga, A., 2012. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Global Ecology and Biogeography 21: 1223.
Baselga, A., D. Orme, S. Villéger, J. De Bortoli, & F. Leprieur, 2018. Betapart: Partitioning beta diversity into turnover and nestedness components. R package version 1.3. Available at: https://cran.r-project.org/web/packages/betapart/.
Bertolo, A., F. G. Blanchet, P. Magnan, P. Brodeur, M. Mingelbier & P. Legendre, 2012. Inferring processes from spatial patterns: the role of directional and non-directional forces in shaping fish larvae distribution in a freshwater lake system. PLoS ONE 7: e50239.
Blanchet, F. G., P. Legendre & D. Borcard, 2008. Modelling directional spatial processes in ecological data. Ecological Modelling 215: 325–336.
Blanchet, F. G., R. Maranger, D. Monti & P. Pepin, 2011. Modelling the effect of directional spatial ecological processes at different scales. Oecologia 166: 357–368.
Borcard, D., P. Legendre & P. Drapeau, 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045–1055. https://doi.org/10.2307/1940179.
Borges, P. P., M. S. Dias, F. R. Carvalho, L. Casatti, P. S. Pompeu, M. Cetra, F. L. Tejerina-Garro, Y. R. Súarez, J. C. Nabout & F. B. Teresa, 2020. Stream fish metacommunity organisation across a Neotropical ecoregion: the role of environment, anthropogenic impact and dispersal-based processes. PLoS ONE 15: e0233733.
Brown, B. L. & C. M. Swan, 2010. Dendritic network structure constrains metacommunity properties in riverine ecosystems. Journal of Animal Ecology 79: 571–580.
Brown, B. L., E. R. Sokol, J. Skelton & B. Tornwall, 2016. Making sense of metacommunities: dispelling the mythology of a metacommunity typology. Oecologia 183: 643–652.
Cardoso, M. R. D., F. F. N. Marcuzzo & J. R. Barros, 2014. Classificação Climática de Köppen-Geiger para o Estado de Goiás e o Distrito Federal. Acta Geographica 8(16): 40–55.
Carrara, F., F. Altermatt, I. Rodriguez-Iturbe & A. Rinaldo, 2012. Dendritic connectivity controls biodiversity patterns in experimental metacommunities. Proceedings of the National Academy of Sciences of the United States of America 109: 5761–5766.
Carvalho, R. A. & F. L. Tejerina-Garro, 2015. Environmental and spatial processes: what controls the functional structure of fish assemblages in tropical rivers and headwater streams? Ecology of Freshwater Fish 24: 317–328.
Carvalho, R. A., H. T. Santana, & F. L. Tejerina-Garro, 2020. Environmental influence on higher fish taxonomic levels: relationships in tropical headwater streams. Studies on Neotropical Fauna and Environment.
Cetra, M., M. Petrere Jr. & W. Barrela, 2017. Relative influences of environmental and spatial factors on stream fish assemblages in Brazilian Atlantic rainforest. Fisheries Management and Ecology 24: 139–145.
Costa e Silva, T., 2015. Revisão taxonômica das espécies do gênero Pimelodus Lacépède, 1803 (Siluriformes: Pimelodidae) na drenagem do rio Tocantins, Brasil. Programa de Pós-Graduação em Ecologia de Ecótonos. Universidade Federal do Tocantins.
Diniz-Filho, J. A. F., T. Siqueira, A. A. Padial, T. F. Rangel, V. L. Landeiro & L. M. Bini, 2012. Spatial autocorrelation analysis allows disentangling the balance between neutral and niche processes in metacommunities. Oikos 121: 201–210.
Dorman, D. F., J. Elith, S. Bacher, C. Buchmann, G. Carl, G. Carré, et al., 2012. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46.
Dray, S. & A. Dufour, 2007. The ade4 Package: implementing the Duality Diagram for Ecologists. Journal of Statistical Software 22(4): 1–20.
Dray, S., D. Bauman, G. Blanchet, D. Borcard, S. Clappe, G. Guenard, et al., 2019. adespatial: Multivariate Multiscale Spatial Analysis. R package version 0.3-7. https://CRAN.R-project.org/package=adespatial.
Erős, T., 2017. Scaling fish metacommunities in stream networks: synthesis and future research avenues. Community Ecology 18: 72–86.
Finn, D. S., N. Bonada, C. Múrria & J. M. Hughes, 2011. Small but mighty: headwaters are vital to stream network biodiversity at two levels of organization. Journal of the North American Benthological Society 30: 963–980.
Frimpong, E. A. & P. L. Angermeier, 2010. Trait-based approaches in the analysis of stream fish communities. American Fisheries Society Symposium 73(109–136): 2010.
Froese, R., & D. Pauly, 2019. FishBase. World Wide Web electronic publication. Available in: www.fishbase.org, version (08/2019).
Gianuca, A. T., S. A. J. Declerck, P. Lemmens & L. Meester, 2017. Effects of dispersal and environmental heterogeneity on the replacement and nestedness components of β-diversity. Ecology 98(2): 525–533.
Gilbert, B. & M. J. Lechowicz, 2004. Neutrality, niches, and dispersal in a temperate forest understory. Proceedings of the National Academy of Sciences of the United States of America 101: 7651–7656.
Grant, E. H. C., W. H. Lowe & W. F. Fagan, 2007. Living in the branches: population dynamics and ecological processes in dendritic networks. Ecology Letters 10: 165–175.
Gravel, D., C. Albouy & W. Thuiller, 2016. The meaning of functional trait composition of food webs for ecosystem function. Philosophical Transactions of Royal Society B 371: 20150268.
Grenouillet, G., D. Pont & C. Herissé, 2004. Within-basin fish assemblage structure: the relative influence of habitat versus stream spatial position on local species richness. Canadian Journal of Fisheries and Aquatic Sciences 61: 93–102.
Heino, J., A. S. Melo, T. Siqueira, J. Soininen, S. Valanko & L. M. Bini, 2015. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater Biology 60: 845–869.
Henriques-Silva, R., M. Logez, N. Reynaud, P. A. Tedesco, S. Brosse, S. R. Januchowski-Hartley, T. Oberdorff & C. Argillier, 2019. A comprehensive examination of the network position hypothesis across multiple river metacommunities. Ecography 42: 284–294.
Holmlund, C. M. & M. Hammer, 1999. Ecosystem services generated by fish populations. Ecological Economics 29: 253–268.
Huang, L., J. Huang, Z. Wu, Y. Mo, Q. Zou, E. Jeppesen & N. Wu, 2019. Beta diversity partitioning and drivers of variations in fish assemblages in a headwater stream: Lijiang River, China. Water 11: 1–16.
Jackson, D. A., P. R. Peres-Neto & J. D. Olden, 2001. What controls who is where in freshwater fish communities—the roles of biotic, abiotic, and spatial factors. Canadian Journal of Fisheries and Aquatic Sciences 58: 157–170.
Keck, B. P., Z. H. Marion, D. J. Martin, J. C. Kaufman, C. P. Harden, J. S. Schwartz & R. J. Strange, 2014. Fish functional traits correlated with environmental variables in a temperate biodiversity hotspot. PLoS ONE 9: e93237.
Kembel, S. W., P. D. Cowan, M. R. Helmus, W. K. Cornwell, H. Morlon, D. D. Ackerly, et al., 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26: 1463–1464.
Legendre, P., & L. Legendre, 2012. Numerical Ecology. 3rd English ed. Elsevier.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Leprieur, F., P. A. Tedesco, B. Hugueny, O. Beauchard, H. H. Dürr, S. Brosse & T. Oberdorff, 2011. Partitioning global patterns of freshwater fish beta diversity reveals contrasting signatures of past climate changes. Ecology Letters 14: 325–334.
Lima, A. C., C. S. Agostinho, D. Sayanda, F. M. Pelicice, A. M. V. M. Soares & K. A. Monaghan, 2016. The rise and fall of fish diversity in a neotropical river after impoundment. Hydrobiologia 763: 207–221.
Logez, M., P. Bady, A. Melcher & D. Pont, 2013. A continental-scale analysis of fish assemblage functional structure in European rivers. Ecography 36: 080–091.
Logue, J. B., N. Mouquet, H. Peter, H. Hillebrand, P. Declerck, A. Flohre, S. Gantner, N. Gülzow, P. Hörtnagl, S. Meier & B. Pecceu, 2011. Empirical approaches to metacommunities: a review and comparison with theory. Trends in Ecology and Evolution 26: 482–491.
Lynch, H. J., E. H. C. Grant, R. Muneepeerakul, M. Arunachalam, I. Rodriguez-Iturbe & W. F. Fagan, 2011. How restructuring river connectivity changes freshwater fish biodiversity and biogeography. Water Resources Research 47: W05531.
Mazzoni, R., N. Fenerich-Verani & E. P. Caramaschi, 2000. Electrofishing a sampling technique for coastal stream fish populations and communities in the southeast of Brazil. Revista Brasileira de Biologia 60: 205–216.
Medeiros, E. S. F., M. J. Silva, B. R. S. Figueiredo, T. P. A. Ramos & R. T. C. Ramos, 2010. Effects of fishing technique on assessing species composition in aquatic systems in semi-arid Brazil. Brazilian Journal of Biology 70: 255–262.
Medina Torres, K. M. M. & C. L. Higgins, 2016. Taxonomic and functional organization in metacommunity structure of stream-fish assemblages among and within river basin in Texas. Aquatic Ecology 50: 247–259.
Meyer, J. L., D. L. Strayer, J. B. Wallace, S. L. Eggert, G. S. Helfman & N. E. Leonard, 2007. The contribution of headwater streams to biodiversity in river networks. Journal of the American Water Resources Association 43: 86–103.
Nimer, E., 1989. Climatologia do Brasil. IBGE, Rio de Janeiro: 421.
Massicotte, P., A. Bertolo, P. Brodeur, C. Hudon, M. Mingelbier & P. Magnan, 2015. Influence of the aquatic vegetation landscape on larval fish abundance. Journal of Great Lakes Research 41: 873–880.
Meffe, G. K. & W. L. Minkley, 1987. Persistence and stability of fish and invertebrate assemblages in a repeatedly disturbed Sonoran Desert stream. American Midland Naturalist 117: 177–191.
Muneepeerakul, R., E. Bertuzzo, H. J. Lynch, W. F. Fagan, A. Rinaldo & I. Rodriguez-Iturbe, 2008. Neutral metacommunity models predict fish diversity patterns in Mississippi-Missouri basin. Nature 453: 220–222.
Münkemüller, T., F. Bello, C. N. Meynard, D. Gravel, S. Lavergne, D. Mouillot, N. Mouquet & W. Thuiller, 2012. From diversity indices to community assembly processes: a test with simulated data. Ecography 35: 468–480.
Nakagawa, H., 2014. Contribution of environmental and spatial factors to the structure of stream fish assemblages at different spatial scales. Ecology of Freshwater Fish 23: 208–223.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, et al., 2019. Package “vegan”. R package version 2.5-6. Available at: https://cran.r-project.org/web/packages/vegan/.
Oliveira, L. G. & P. C. Bispo, 2001. Ecologia de comunidades das larvas de Trichoptera Kirby (Insecta) em dois córregos de primeira ordem da Serra dos Pirineus, Pirenópolis, Goiás, Brasil. Revista Brasileira de Zoologia 18: 1245–1252.
Pavoine, S. & M. B. Bonsall, 2010. Measuring biodiversity to explain community assembly: a unified approach. Biological Reviews 86: 792–812.
Pavoine, S., J. Vallet, A.-B. Dufour, S. Gachet & H. Daniel, 2009. On the challenge of treating various types of variables: application for improving the measurement of functional diversity. Oikos 118: 391–402.
Pease, A. A., A. A. González-Díaz, R. Rodiles-Hernández & K. O. Winemiller, 2012. Functional diversity and trait–environment relationships of stream fish assemblages in a large tropical catchment. Freshwater Biology 5: 1060–1075.
Pease, A. A., M. Mendoza-Carranza & K. O. Winemiller, 2018. Feeding ecology and ecomorphology of cichlid assemblages in a large Mesoamerican river delta. Environmental Biology of Fishes 101: 867–879.
Peláez, O. E., F. M. Azevedo & C. S. Pavanelli, 2017. Environmental heterogeneity explains species turnover but not nestedness in fish assemblages of a Neotropical basin. Acta Limnologica Brasiliensia 29: e117.
Peláez, O. E. & C. S. Pavanelli, 2019. Environmental heterogeneity and dispersal limitation explain different aspects of β-diversity in Neotropical fish assemblages. Freshwater Biology 64: 497–505.
Peres-Neto, P. R. & P. Legendre, 2010. Estimating and controlling for spatial structure in the study of ecological communities. Global Ecology and Biogeography 19: 174–184.
Poff, N. L., 1997. Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. Journal of the North American Benthological Society 16: 391–409.
Pool, T. K., G. Grenouillet & S. Villéger, 2014. Species contribute differently to the taxonomic, functional, and phylogenetic alpha and beta diversity of freshwater fish communities. Diversity and distributions 20: 1235–1244.
R Core Team. 2019. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. URL https://www.R-project.org/.
Roa-Fuentes, C. A., L. Casatti & R. M. Romero, 2015. Phylogenetic signal and major ecological shifts in the ecomorphological structure of stream fish in two river basins in Brazil. Neotropical Ichthyology 13: 165–178.
Rocha, M. S., 2012. Sistemática da família Pimelodidae Swainson, 1838 (Teleostei: Siluriformes). Instituto Nacional de Pesquisas da Amazônia, INPA.
Rodrigues-Filho, C. A. S., R. C. Gurgel-Lourenço, L. A. V. Bezerra, E. F. Oliveira, R. P. Leitão, D. S. Garcez & J. L. Sánchez-Botero, 2018. How are local fish communities structured in Brazilian semiarid headwater streams? Hydrobiologia 819: 93–108.
dos Santos, G. M., B. de Mérona, A. A. Juras & M. Jégu, 2004. Peixes do baixo rio Tocantins: 20 anos depois da usina hidrelétrica Tucuruí. Eletronorte, Brasília, DF.
Soininen, J., 2016. Spatial structure in ecological communities—a quantitative analysis. Oikos 125: 160–166.
Sharma, S., P. Legendre, M. Cáceres & D. Boisclair, 2011. The role of environmental and spatial processes in structuring native and non-native fish communities across thousands of lakes. Ecography 34: 762–771.
Strecker, A. L., J. D. Olden, J. B. Whittier & C. P. Paukert, 2011. Defining conservation priorities for freshwater fishes according to taxonomic, functional, and phylogenetic diversity. Ecological Applications 21: 3002–3013.
Stuart-Smith, R., A. Bates, J. Lefcheck, et al., 2013. Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature 501: 539–542.
Súarez, Y. R. & M. Petrere Jr., 2006. Gradientes de diversidade nas comunidades de peixes da bacia do rio Iguatemi, Mato Grosso do Sul, Brasil. Iheringia 96: 197–204.
Tejerina-Garro, F. L., 2008. Biodiversidade e impactos ambientais no estado de Goiás. In Rocha, C., F. L. Tejerina-Garro & J. P. Pietrafesa (eds), Cerrado, Sociedade e Meio Ambiente: Desenvolvimento Sustentável em Goiás. Editora da Universidade Católica de Goiás, Goiânia, GO: 15–47.
Tejerina-Garro, F. L. & B. Mérona, 2000. Gill net sampling standardisation in large rivers of French Guiana (South America). Bulletin Français de La Pêche et de La Pisciculture 357(358): 227–240.
Tejerina-Garro, F. L., M. Maldonado, C. Ibañez, D. Pont, N. Roset & T. Oberdorff, 2005. Effects of natural and anthropogenic environmental changes on riverine fish assemblages: a framework for ecological assessment of rivers. Brazilian Archives of Biology and Technology 48: 91–108.
Tejerina-Garro, F. L., R. A. Carvalho & F. B. Teresa, 2017. A biodiversidade e a conservação da ictiofauna do alto da bacia do rio Paraná no estado de Goiás, Brasil Central. In Hannibal, W., R. F. Rossi, I. L. Morais & L. H. M. Teixeira (eds), Biodiversidade, Manejo e Conservação do Sul de Goiás. Paco Editorial, Jundiaí, SP: 93–126.
Tonkin, J. D., F. Altermatt, D. S. Finn, J. Heino, J. D. Olden, S. U. Pauls & D. A. Lytle, 2018. The role of dispersal in river network metacommunities: patterns, processes, and pathways. Freshwater Biology 63: 141–163.
Villéger, S., S. Brosse, M. Mouchet, D. Mouillot & M. J. Vanni, 2017. Functional ecology of fish: current approaches and future challenges. Aquatic Sciences 79: 783–801.
Vitorino Jr., O. B., R. Fernandes, C. S. Agostinho & F. M. Pelicice, 2016. Riverine networks constrain β-diversity patterns among fish assemblages in a large Neotropical river. Freshwater Biology 61: 1733–1745.
Wiens, J. A., 1989. Spatial scaling in ecology. Functional Ecology 3: 385–397.
Winemiller, K. O., 1989. Patterns of variation in life history among South American fishes in seasonal environments. Oecologia 81: 225–241.
Winemiller, K. O., 2005. Life history strategies, population regulation, and implications for fisheries management. Canadian Journal of Fisheries and Aquatic Sciences 62: 872–885.
Zbinden, Z. D. & W. J. Matthews, 2017. Beta diversity of stream fish assemblages: partitioning variation between spatial and environmental factors. Freshwater Biology 62: 1460–1471.
Zuur, A. F., E. N. Ieno & C. S. Elphick, 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14.
Acknowledgements
We thank FAPEG for the financial support to the Project #14597. We thank Waldeir Francisco de Menezes and Nicelly Braudes de Araújo for their help with data collection in field and the extensive work at the laboratory. We also thank reviewers for comments that helped to improve the manuscript. FBT is supported by CNPq (306912/2018-0).
Author information
Authors and Affiliations
Contributions
RAC and FLTG were responsible for study design and fish species sampling. RAC, FLTG, and FBT were responsible for statistical analyses and manuscript writing. All authors read the manuscript and gave their final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Fernando M. Pelicice
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carvalho, R.A., Teresa, F.B. & Tejerina-Garro, F.L. The effect of riverine networks on fish β-diversity patterns in a Neotropical system. Hydrobiologia 848, 515–529 (2021). https://doi.org/10.1007/s10750-020-04459-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04459-9