Abstract
Urban stormwater ponds are important for flood protection and provide habitat for plants and animals in heavily sealed cities. Little is known about the diversity of plants in urban stormwater ponds and the vegetation composition is often influenced by sowing and planting. We analysed the re-colonisation of the vegetation in an urban stormwater pond, which was reconstructed to improve water retention. Specifically, we studied if the soil seed bank has the potential for re-colonising the pond. We analysed the standing vegetation from the year before until 2 years after reconstruction of a stormwater pond in Hamburg, Germany. Further, we analysed the soil seed bank in the year before and in the second year after reconstruction of the stormwater pond. We found 74 species in the soil seed bank in 2015 and 2017 with Juncus spp. and Epilobium spp. being the most dominant taxa. Our results indicate that urban stormwater ponds have the potential for re-colonisation out of seed bank and, thus, sowing is not a necessary management action in the reconstruction process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Land-use change is projected to be one of the major factors for biodiversity change in terrestrial and aquatic ecosystems worldwide by the year 2100 (Sala et al., 2000) with urbanisation being one specific process of it. Urban areas are characterised by altered environmental conditions in comparison to non-urban areas. For instance, in Hamburg, Germany, the so-called ‘urban heat island effect’ leads to higher mean temperatures up to 1.1 °C in comparison to non-urban areas (Schlünzen et al., 2010). Even more important, the sealing of the soil surface leads to a direct loss of habitats for plants and to a loss of biodiversity. The soil available for plant colonisation often contains high amounts of nutrients and pollutants. Hydrological conditions of soils in urban areas are also altered with a highly reduced infiltration capacity of the soil due to soil sealing. As a consequence, runoff is not able to seep away and the risk of floods increases with associated threats for human life and for physical damage of buildings and further infrastructure (Pickett et al., 2001; Scalenghe & Marsan, 2009; Depietri et al., 2012). Therefore, soil sealing may have strong effects on stream hydrology. In heavily sealed areas, heavy rainfall can lead to increased discharges of streams resulting potentially in flooding of nearby areas (Smith et al., 2005). Constructed stormwater ponds are attempting to mitigate these negative effects (Bell et al., 2016).
Stormwater ponds are usually of anthropogenic origin and an important part of flood protection strategies in heavily sealed areas due to their capacity for temporal storage of runoff after heavy rainfalls. Here, the term ‘stormwater pond’ is used in the context of describing all different types of ponds available for intermediate storage of water, regardless of the source of water (flood waters or rain waters) and the hydrological conditions of the stormwater pond (wet, moist and/or dry conditions). In addition to the primary function of water storage, urban stormwater ponds can also act as sediment and nutrient traps, they can be used by people for recreational purposes and provide habitat for plants and animals (Lundy & Wade, 2011; Moore & Hunt, 2012). Overall, stormwater ponds are dynamic ecosystems characterised by changing water tables in time and space. The frequency and duration of flooding events and the groundwater level below soil surface of a stormwater pond are important factors determining the composition of its flora (Casanova & Brock, 2000; Fan et al., 2013).
The importance of stormwater ponds for urban biodiversity of animals is well documented, mostly for aquatic animals or animals with a life cycle partly depending on aquatic conditions. Stormwater ponds can contain diverse odonate assemblages and promote a higher richness of threatened odonate species due to a higher habitat quality than water bodies without retention function (Scher & Thièry, 2005; Holtmann et al., 2018). Diverse communities of other invertebrates (Briers, 2014; Hassall & Anderson, 2015) and amphibians (Hamer et al., 2012; Le Viol et al., 2012) are also documented for stormwater ponds. High bat activity above stormwater ponds in an agricultural landscape indicates the importance of stormwater ponds also for non-aquatic animals (Stahlschmidt et al., 2012). In general, the value of stormwater ponds for animal biodiversity depends on the abiotic and biotic conditions of the pond and the surrounding area as well as on the considered animal group (Le Viol et al., 2012; Gallagher et al., 2014; Holtmann et al., 2017). In contrast to animal biodiversity, surprisingly little is known about the importance of stormwater ponds for the biodiversity of wild plants.
Due to their dynamic and small-scale heterogeneity, stormwater ponds can harbour plant species adapted to different environmental conditions, such as macrophytes in permanent flooded areas or plant species adapted to mesic water conditions in drier parts of a stormwater pond. During flooding, sedimentation and erosion processes may disturb vegetation cover and subsequently, bare soil patches can develop. These areas can be recolonised by plants out of the soil seed bank or due to input via dispersal processes, mainly input via hydrochory (Henry et al., 1996; Abernethy & Willby, 1999; Barrat-Segretain & Bornette, 2000; Vogt et al., 2004). Therefore, stormwater ponds could be a secondary habitat for many wild plant species of aquatic to mesic habitats. This is especially promising in urban areas, where, e.g. plant species adapted to aquatic conditions are at high extinction risk (Duncan et al., 2011). For bryophytes it is known that, species adapted to less shaded, bare soils and moist water conditions can be found in stormwater ponds. Furthermore, some of these bryophyte species are threatened and characterised by the ability to develop persistent spores or bulbils. As a consequence, these species are able to survive periods with unfavourable environmental conditions in the soil of stormwater ponds (Solga, 2001). Stormwater ponds may even contain more aquatic and threatened vascular plant species than ponds without retention function due to their higher habitat heterogeneity, which highlights the importance of stormwater ponds as habitat for vascular plants (Holtmann et al., 2019). However, knowledge about the re-colonisation ability of vascular plant species in stormwater ponds is scarce.
Seeds of different species differ in their ability to persist in the soil and to build a seed bank (Thompson et al., 1997). The longevity of seeds is affected by internal factors such as dormancy type, resistance against pathogens or seed form and size, and by external factors such as soil texture, temperature or presence of pathogens or granivores (Jensen, 2004). In natural ecosystems, persistent seed banks can often be found in dynamic habitats, such as wetlands (Hopfensperger, 2007), but as far as we know, this is the first study about the seed bank of an urban stormwater pond.
In the city of Hamburg, seeds are usually sown on the soils of newly created or reconstructed stormwater ponds to facilitate fast re-colonisation and, thereby, prevent soil erosion. Sowing seeds may inhibit natural vegetation development and a homogenous vegetation cover may develop in these stormwater ponds. As reported for intensively used grasslands, a low plant biodiversity after sowing can be the consequence (Batáry et al., 2012). In other cities, such as Münster (Germany), sowing and planting of plants is not performed in stormwater ponds (Holtmann et al., 2019), and therefore, natural vegetation development can occur in these stormwater ponds. In addition, the environmental conditions before the creation or reconstruction of a stormwater pond as well as the planning targets and actions during the construction process, such as removing of pavement or input of soil, influence the development of vegetation in stormwater ponds. Knowledge about the re-colonisation ability of seeds in the soils of stormwater ponds is important for decision-makers and the planning and construction of stormwater ponds to assess if the soil seed bank would be sufficient for the colonisation process.
The vegetation of an urban stormwater pond in Hamburg was studied for a period of 3 years. In 2016, the stormwater pond was reconstructed due to the development of a wet forest as a consequence of a missing management and flooding in the past. The soil of the stormwater pond was not completely replaced, which could allow the vegetation to recolonise out of the soil seed bank. We analysed the standing herbaceous vegetation of the stormwater pond over a period of 3 years, starting in the year before the reconstruction and furthermore, the seed bank was studied in the year before and in the second year after the reconstruction. For gaining information on the re-colonisation ability in the channel, plain, lower and upper shoreline of the stormwater pond, these four zones characterised by different hydrological conditions were studied. We aimed at answering the following research questions: Has the soil seed bank of the stormwater pond the potential for a natural re-colonisation of the bare soil after reconstruction? Which species profit from the colonisation from the seed bank in the different zones of the stormwater pond? How does species richness develop after reconstruction of the stormwater pond?
Materials and methods
Study object
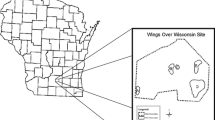
The stormwater pond is located in the city of Hamburg in northern Germany (Fig. 1a). Hamburg has about 1.8 million inhabitants and covers an area of 755 km2 (Statistische Ämter des Bundes und der Länder, 2018). The climate of Hamburg is temperate and oceanic with a mean annual temperature of 9.4 °C and an annual precipitation of 793 mm (DWD, 2019; reference period 1981–2010). The city is dominated by settlement (47%) and agricultural areas (23%) and furthermore, by traffic areas (12%), water bodies (8%) and woodlands (8%; Statistische Ämter des Bundes und der Länder, 2018).
Location of the studied stormwater pond ‘Farnhornstieg’ (53°35′31″N, 9°54′30″E) in the city of Hamburg in northern Germany (a, b) and zones (channel, plain, lower and upper shoreline, wet forest) of the stormwater pond based on the planning documents before the reconstruction of the stormwater pond (c)
The stormwater pond ‘Farnhornstieg’ (53°35′31″N, 9°54′30″E) is located in the urban district Eidelstedt in the north-western part of Hamburg (Fig. 1b). Until 1998, the property was used for storage, but after demolition of the warehouse and soil remediation, the area was used for water retention of the nearby stream ‘Mühlenau’ to prevent flooding of the nearby areas. Due to a missing management of the vegetation, a wet forest composed of black alder (Alnus glutinosa (L.) Gaertn.) and different willow species (Salix spp.) established and the function for water retention was gradually reduced due to a decreased volume for retention (Fig. 2). In spring 2016, the stormwater pond was reconstructed to improve the retention of stormwater from the ‘Mühlenau’ by removing the wet forest in most parts of the stormwater pond and regrading of the stormwater pond. To prevent soil erosion, most of the bare soil of the stormwater pond was sown with different seed mixtures (grasses and herbs) in the beginning of 2016, dependent on the hydrological conditions of the soil. The local authorities instructed a commercial company to use plant species for seed sowing from a list with recommended species for sowing measures (FLL, 2016), but details on the used plant species in the seed mixtures were not documented. Furthermore, some species adapted to wetland conditions were planted in the channel and along the shorelines of the stormwater pond. Sandy substrates mixed with demolition waste and with soil organic matter < 1% can be found in the stormwater pond. From July 2016 to December 2017, the water content of the soil ranged from 346.67 to 409.86 mm until 1 m soil depth (mean: 379.40 mm until 1 m soil depth) and the water level fluctuated between 17.87 to 90.79 cm a.s.l. (above sea level; mean: 35.24 cm a.s.l.; Kalinski, unpublished data).
Orthophotos of the stormwater pond ‘Farnhornstieg’ in Hamburg. Pictures show the vegetation development of the stormwater pond in summer 2013, spring and summer 2016 and spring 2018. In summer 2013, the stormwater pond was dominated by a dense wet forest composed of black alder (Alnus glutinosa (L.) Gaertn.) and different willow species (Salix spp.). After the reconstruction, in spring 2016, wheel tracks of the construction machines are still visible and the soil is still unvegetated. In summer 2016 and spring 2018, the vegetation in the stormwater pond has developed. Orthophotos: Landesbetrieb Geoinformation und Vermessung, Hamburg (2020)
Vegetation mapping and seed bank analysis
The vegetation of the stormwater pond was studied in late summer (August, September) 2015, 2016 and 2017. For each zone (channel, plain, lower and upper shoreline; Fig. 1c), five randomly distributed 1 m2-plots were selected to gain a balanced study design. The relatively small plot size of 1 m2 was selected to guarantee sampling of homogenous vegetation of the zones channel, lower shoreline and upper shoreline, whose areas are partly very small. In 2015, the plots were marked with a handheld GPS device and after reconstruction in 2016, we additionally marked the plots with soil magnets. The soil magnets guaranteed the precise identification of the study plots. The wet forest in the western part of the pond was not studied, due to the inaccessibility of this area and a missing herbaceous vegetation layer as a result of permanently standing water. All herbaceous vascular plant species rooting in the plots or reaching with their aboveground parts into the plots were recorded with their coverages according to a modified scale of Braun-Blanquet after Dierschke (1994; r = 0.1%, + = 0.5%, 1 = 2.5%, 2 m = 2.5%, 2a = 8.8%, 2b = 20.0%, 3 = 37.5%, 4 = 62.5%, 5 = 87.5%).
On 11 August 2015 and 08 September 2017, mixed soil samples were collected from each study plot by using a soil steel corer (diameter: 50 mm, depth: 51 mm), combining five samples from the four corners and one from the centre of each plot. The soil samples were stratified in darkness for approx. 17 weeks at 4 °C and afterwards processed according to Ter Heerdt et al. (1996). The soil samples were concentrated under running water using two stacked soil sieves (mesh size: 4.0 mm and 0.212 mm). The plant material < 0.4 and > 0.212 mm was used for analysing the seed bank by spreading it into trays on a 2 cm thick layer of a compost-sand-substrate (1:1 by volume). The 0.4 mm sieve was used for removing vegetative plant material as it could re-sprout and the 0.212 mm sieve was used to minimise soil volume by eliminating the clay fraction, while the smallest expectable seeds of Juncus species were retained. On 14 November 2015 and 18 January 2018, the trays were put in a greenhouse at 20/15 °C (day/night) and a light period of 12 h/day. Additional trays with only substrate as controls were put in the greenhouse to capture seeds included in the substrate and seed input through air. The samples were watered regularly and kept moist. All emerging herbaceous seedlings were continuously counted, identified and afterwards removed from the trays. Plant determination was done using Csapody (1968), Raabe (1975), Hanf (1990), Christensen (1999) and Jäger (2017). Seedlings which could not be identified at this early life stage were transferred to pots and cultivated in the greenhouse for later identification. The species Epilobium ciliatum Raf. + Epilobium obscurum Schreb. + Epilobium parviflorum Schreb., Juncus bufonius L. + Juncus effusus L. and Persicaria lapathifolia (L.) Delarbre + Persicaria maculosa Gray + Persicaria mitis (Schrank) Assenov were treated as one taxon each (Epilobium spp., Juncus spp. and Persicaria spp.) due to high numbers of seedlings and difficult determination of seedlings. When no new seedlings occurred in the trays for 7 days, the watering of the trays was suspended and the soil dried out. After 3 weeks, the hard soil layer was crumbled and again watered regularly for initiating additional germination of seeds from deeper soil layers. When no new seedlings emerged in the trays after drying for 7 days, the analysis was terminated. Seedlings which could not be identified to species level were identified to genus or family level. The nomenclature of plant species follows Jäger (2017).
Data preparation and statistical analysis
Analyses were performed with species groups like Oenothera biennis agg. or Taraxacum sect. Ruderalia Kirschner, H. Øllg. & Štěpánek treated as species. In all analyses, the species groups Epilobium spp., Juncus spp. and Persicaria spp. were used for comparison between the seed bank and the standing vegetation. Epilobium hirsutum L. was excluded from further analysis of the seed bank due to its occurrence in the control trays in both years. Classification of species as threatened species for Hamburg was derived from Poppendieck et al. (2010). For the standing vegetation, the total number of species per plot and year was calculated.
Detrended correspondence analysis (DCA) was used to detect vegetation patterns of the seed bank and standing vegetation of the study plots. To be able to compare species composition between the standing vegetation (cover) and the seed bank (number of seeds), we transformed the data to relative abundance (vegetation: \(\frac{\text{cover of each species}}{\text{cover sum of all species}} \times 100\); seed bank: \(\frac{\text{number of seeds of each species}}{\text{sum of total number of seeds}} \times 100\)) for each plot. In total, 117 species and 95 plots were considered in the DCA, while rare species were down-weighted in the analysis. Five plots could not be considered in the DCA as no vegetation was present in theses plots. Species acronyms were generated from the three first characters from the genus and specific epithet of each species. Furthermore, Sørensen distance values were calculated as similarity index for comparing the species compositions of the standing vegetation and the seed bank for each plot for the years 2015 and 2017. For two plots no Sørensen distance values could be calculated as no vegetation was present in these plots.
We used repeated measures ANOVA (analysis of variance) to test for significant differences in the total number of species per plot between zones and years. To meet the assumptions of normal distribution and homogeneity of variance, we performed a box-cox-transformation of data (total number of species: λ = 0.32). Normal distribution and homogeneity of variance were checked visually using QQ-plot. ANOVA with significant result was followed by Tukey HSD post hoc test to identify significant differences between the years for each zone.
Box-cox-transformation of data, QQ-plot, repeated measures ANOVA and post hoc test were carried out using STATISTICA 12.0 (StatSoft Inc., 2014), while ordination and calculation of Sørensen distance values was performed using PC-ORD 7.04 (McCune & Mefford, 2006).
Results
The DCA revealed more homogeneous species compositions in the standing herbaceous vegetation of the plain and upper shoreline as these plots cluster in the ordination diagram in comparison to the plots of the channel and lower shoreline, which are scattered across the left side of the ordination diagram (Fig. 3a). The species composition of the standing vegetation of the zones remained relatively stable over the three study years (Fig. 3a, b) indicating that the sowing did not affect the species composition of the herbaceous vegetation. The vegetation of the upper and lower shoreline developed in the direction of the other zones in 2016, but in 2017 the species composition was already close to the composition in 2015 (Fig. 3b). The soil seed bank compositions grouped quite close together in between the channel and plain, and the upper and lower shoreline plots of the standing vegetation (Fig. 3a). The visible low similarity between the species composition of the soil seed bank and the species composition of the standing vegetation was also indicated by high Sørensen distance values (0.89 ± 0.02; mean ± standard error). The species composition of the seed bank differed between the two study years, as the samples from 2017 are clustering very close together (Fig. 3b) The species are ordered along axis 1 indicating a moisture gradient of the study plots, with dry meadow species located in the left part of the diagram and hydrophytes and macrophytes located in the right part of the diagram (Fig. 3c). In the soil seed bank, Glyceria declinata Bréb, Senecio erraticus Bertol. and Senecio sylvaticus L. were identified as threatened species for Hamburg and additional, Hypericum tetrapterum Fr. was found as threatened species in the standing vegetation. Polypogon monspeliensis (L.) Desf. was identified in the soil seed bank in 2015 as an unrecorded species for the city of Hamburg (Supplementary Material 1).
Detrended correspondence analysis (DCA) of the studied stormwater pond based on 95 studied plots (a, b) and 117 herbaceous plant species (c) found in the soil seed bank and standing vegetation. The species are ordered along axis 1 indicating a moisture gradient of the study plots, with dry meadow species located in the left part of the diagram and hydrophytes and macrophytes located in the right part of the diagram. The soil seed bank compositions group quite close together in between the channel and plain, and the upper and lower shoreline plots of the standing vegetation. Study plots are differentiated after type of data (soil seed bank = open symbols, standing vegetation = filled symbols; a, b, zones (channel = triangle, plain = circle, lower shoreline = square, upper shoreline = reverse triangle; a and year (2015 = square, 2016 = triangle, 2017 = circle; b. Species acronyms were generated from the three first characters from the genus and specific epithet of each species (compare Supplementary Material 1). Axis 1: eigenvalue 0.88, gradient length 12.01 SD; axis 2: eigenvalue 0.76, gradient length 5.15 SD
In the soil seed bank, we recorded 174,174 ± 70,235 seeds m−2 in 2015 and 72,050 ± 13,522 seeds m−2 in 2017. The most frequent species in the soil seed bank in both years were Juncus spp. (2015: 162,816 ± 69,878 seeds m−2, 2017: 53,069 ± 11,540 seeds m−2) and Epilobium spp. (2015: 581 ± 373 seeds m−2, 2017: 7553 ± 3468 seeds m−2). The number of seeds m−2 of Juncus spp. in the soil seed bank of the channel and plain decreased strongly from 2015 to 2017 (Supplementary Material 1), whereas the number of seeds m−2 increased slightly in the lower and upper shoreline. In contrast, the number of seeds m−2 of Epilobium spp. in the soil seed bank increased in all zones from 2015 to 2017 with the strongest increase in the plain from 489 ± 292 seeds m−2 in 2015 to 15,381 ± 9083 seeds m−2 in 2017. Juncus spp. was identified in the standing vegetation in all study years, but especially in the plain plots, an increase of the coverages from 2015 (5 ± 4%) to 2016 and 2017 (both 36 ± 7%) was recorded. The same pattern was found for Epilobium spp. with a minor increase of the coverages. Additional species with high numbers of seeds m−2 in the seed bank in 2015 were Arabidopsis thaliana (L.) Heynh., Gnaphalium uliginosum L., Hypericum perforatum L., Lythrum salicaria L., Oenothera biennis agg., Poa palustris L., Rumex acetosa L., Sagina procumbens L. and Urtica dioica L.. Lythrum salicaria and Oenothera biennis agg. were recorded with high coverages in the standing vegetation after the reconstruction, even though only in one year or one zone. A decrease of the coverages after the reconstruction was recorded for Urtica dioica. Gnaphalium uliginosum was identified in the standing vegetation only in the year 2016. Hypericum perforatum and Poa palustris were identified in the standing vegetation after the reconstruction, but with low coverages. Arabidopsis thaliana and Sagina procumbens were not identified in the standing vegetation after the reconstruction.
In total, 74 species were found in the soil seed bank of all zones in both years, with more species (60 species) found in the second year after the reconstruction in comparison to the year before the reconstruction (51 species). In the standing vegetation, 95 species (using the same species groups as for the seed bank) were identified in the study plots in all 3 years (Supplementary Material 1). After sowing in the beginning of 2016, the total number of species of all plots was highest with 77 species in summer 2016, while the total number of species was considerably lower in the year before the reconstruction (2015: 37 species) and in the second year after reconstruction (2017: 57 species). Except for the channel, the number of species in the standing vegetation was significantly lower in 2015 in comparison to 2016 (Fig. 4). In all these three zones, the lowest number of species was found in 2015. However, the number of species was not significantly different in the years 2016 and 2017 (Tukey HSD post hoc test).
Number of species of the study plots of the standing herbaceous vegetation of the stormwater pond ‘Farnhornstieg’ in Hamburg, Germany, for the years 2015, 2016 and 2017, grouped according to the different zones of the stormwater pond (channel, plain, lower shoreline and upper shoreline). Letters indicate significant differences (p < 0.05) between the years for the different zones after Tukey HSD post hoc test using box-cox-transformed data. Results of repeated measures ANOVA: zone: F = 57.8, p < 0.001; year: F = 17.3, p < 0.001; zone * year: F = 9.8, p < 0.001. Mean and standard error of the original data are shown
Discussion
Our study showed that the soil seed bank of an urban stormwater pond has the potential for natural re-colonisation after reconstruction indicated by high seed numbers in the soil seed bank. These high numbers of seeds m−2 in both years are consistent with known seed densities of different types of wetlands: 15,450 seeds m−2 in a floodplain (Abernethy & Willby, 1999), 56,000 seeds m−2 in a wet meadow (Jensen, 1998) and 225,756 seeds m−2 in an ephemeral wetland (Bissels et al., 2005). The most dominant species group in the seed bank in both study years was Juncus spp. (Juncus bufonius, J. effusus). Juncus species are known to have a high seed production leading to high seed rains and to build persistent seed banks (Thompson et al., 1997; Jensen, 1998; Ervin & Wetzel, 2001). The high number of Juncus seeds recorded in the seed bank of the stormwater pond in both study years is in accordance with other studies. Juncus species were found to be the most abundant species with high seed densities in the seed bank of different types of wet habitats (Jensen, 1998; Bissels et al., 2005; Bossuyt & Honnay, 2008). Therefore, the bare soils of the stormwater pond could be colonised quickly and, as vegetation dominated by Juncus species may not inhibit germination of other herbaceous species, a more heterogeneous vegetation could develop over time (Ludewig et al., 2015). For the second most abundant species group in our seed bank study, Epilobium spp. (Epilobium ciliatum, E. obscurum, E. parviflorum), persistent seed banks were reported as well (Thompson et al., 1997). Persistent seed banks are supportive for species to cope with changing environmental conditions or disturbance events and thus persistent seed banks are an important feature in dynamic ecosystems with unpredictable conditions (Capon & Brock, 2006; Brock, 2011). The low similarity between the species composition of the soil seed bank and the species composition of the standing vegetation in our study, is in accordance with other wetland studies where the similarity of the soil seed bank and the standing vegetation was low as well (Jensen, 1998; Hopfensperger, 2007; Hopfensperger et al., 2009; Kimura & Tsuyuzaki, 2011).
Two main groups of plant species were found in the soil seed bank: species of different types of wet habitats (such as reeds or wet meadows) and ruderal species. Germination requirements of wetland species vary among species, but in general, wetland species are known to germinate under a variety of different environmental conditions. This includes hydrological conditions ranging from moist or flooded soils to dry soils, wide temperature ranges and different light conditions. Furthermore, species also differ in their requirements for dormancy breaking (Keddy & Ellis, 1985; Schütz, 2000; Boedeltje et al., 2002; Brändel, 2006; Ludewig et al., 2014). When the species composition of wetland species in the seed bank is diverse, it could be assumed that the germination requirements of at least some species could be fulfilled. Further, we found ruderal species in the soil seed bank, which are able to recolonise rapidly after disturbance (Grime, 1979). The increase in the coverages of Juncus species from 2015 to 2017, the high coverages of Juncus and Epilobium species and the occurrence of ruderal species in the standing vegetation in the first and second year after reconstruction indicate that these species benefit from natural re-colonisation. Accordingly, species richness was highest in 2016 in all zones except for the channel, which shows that some species profit from activating the seed bank and probably from sowing. It can be assumed that the composition of the standing vegetation will change in the future, depending on the frequency and duration of flooding. When the hydrological conditions in the channel and plain will become drier in the future, germination and establishment of species adapted to drier conditions, such as Arabidopsis thaliana or Cardamine hirsuta L., may occur. This resilience of the soil seed bank to react to changing environmental conditions is used in restoration projects to initiate former vegetation composition of wetlands, but the success of re-establishing former vegetation out of the seed bank depends on the initial conditions (Brouwer & Roelofs, 2001; Roelofs et al., 2002; Beas et al., 2013; Bauer et al., 2018).
Conclusion
Urban stormwater ponds can contain high numbers of seeds in the soil seed bank from diverse vascular plant species concerning lifeforms and preferred habitats, highlighting the potential for natural re-colonisation after reconstruction and thus preventing soil erosion as a consequence of flooding. Therefore, we recommend testing natural re-colonisation after the reconstruction of stormwater ponds or other constructed wetlands. While sowing (and planting) of plant species does not seem to be necessary in the construction process (indicated by relatively stable species compositions of the standing vegetation of the zones over the three study years), it may be helpful if specific plant species are favoured. Overall, natural re-colonisation would possibly promote a higher habitat heterogeneity and thus higher biodiversity than sowing and should, therefore, be preferred in areas for construction of stormwater ponds, where soil seed banks are present (Holtmann et al., 2019).
References
Abernethy, V. J. & N. J. Willby, 1999. Changes along a disturbance gradient in the density and composition of propagule banks in floodplain aquatic habitats. Plant Ecology 140: 177–190.
Barrat-Segretain, M.-H. & G. Bornette, 2000. Regeneration and colonization abilities of aquatic plant fragments: effect of disturbance seasonality. Hydrobiologia 421: 31–39.
Batáry, P., A. Holzschuh, K. M. Orici, F. Samu & T. Tscharntke, 2012. Responses of plant, insect and spider biodiversity to local and landscape scale management intensity in cereal crops and grasslands. Agriculture, Ecosystems and Environment 146: 130–136.
Bauer, M., R. Harzer, K. Strobl & J. Kollmann, 2018. Resilience of riparian vegetation after restoration measures on River Inn. River Research and Applications 34: 451–460.
Beas, B. J., L. M. Smith, K. R. Hickman, T. G. LaGrange & R. Stutheit, 2013. Seed bank responses to wetland restoration: do restored wetlands resemble reference conditions following sediment removal? Aquatic Botany 108: 7–15.
Bell, C. D., S. K. McMillan, S. M. Clinton & A. J. Jefferson, 2016. Hydrologic response to stormwater control measures in urban watersheds. Journal of Hydrology 541: 1488–1500.
Bissels, S., T. W. Donath, N. Hölzel & A. Otte, 2005. Ephemeral wetland vegetation in irregularly flooded arable fields along the northern Upper Rhine: the importance of persistent seedbanks. Phytocoenologia 35: 469–488.
Boedeltje, G., G. N. J. Ter Heerdt & J. P. Bakker, 2002. Applying the seedling-emergence method under waterlogged conditions to detect the seed bank of aquatic plants in submerged sediments. Aquatic Botany 72: 121–128.
Bossuyt, B. & O. Honnay, 2008. Can the seed bank be used for ecological restoration? An overview of seed bank characteristics in European communities. Journal of Vegetation Science 19: 875–884.
Brändel, M., 2006. Effect of temperatures on dormancy and germination in three species in the Lamiaceae occurring in northern wetlands. Wetlands Ecology and Management 14: 11–28.
Briers, R. A., 2014. Invertebrate communities and environmental conditions in a series of urban drainage ponds in Eastern Scotland: implications for biodiversity and conservation value of SUDS. Clean - Soil, Air, Water 42: 193–200.
Brock, M. A., 2011. Persistence of seed banks in Australian temporary wetlands. Freshwater Biology 56: 1312–1327.
Brouwer, E. & J. G. M. Roelofs, 2001. Degraded softwater lakes: possibilities for restoration. Restoration Ecology 9: 155–166.
Capon, S. J. & M. A. Brock, 2006. Flooding, soil seed bank dynamics and vegetation resilience of a hydrologically variable desert floodplain. Freshwater Biology 51: 206–223.
Casanova, M. T. & M. A. Brock, 2000. How do depth, duration and frequency of flooding influence the establishment of wetland plant communities? Plant Ecology 147: 237–250.
Christensen, E., 1999. Bestimmungshilfen für Seggen und vegetativ ähnliche Riedgräser des norddeutschen Flachlandes im blütenlosen Zustand - Teil 2. Rundbrief zur botanischen Erfassung des Kreises Plön (Nord-Teil) 8: 2–16.
Csapody, V., 1968. Keimlingsbestimmungsbuch der Dikotyledonen. Akadémiai Kiadó, Budapest.
Depietri, Y., F. G. Renaud & G. Kallis, 2012. Heat waves and floods in urban areas: a policy-oriented review of ecosystem services. Sustainability Science 7: 95–107.
Dierschke, H., 1994. Pflanzensoziologie. Verlag Eugen Ulmer, Stuttgart.
Duncan, R. P., S. E. Clemants, R. T. Corlett, A. K. Hahs, M. A. McCarthy, M. J. McDonnell, M. W. Schwartz, K. Thompson, P. A. Vesk & N. S. G. Williams, 2011. Plant traits and extinction in urban areas: a meta-analysis of 11 cities. Global Ecology and Biogeography 20: 509–519.
DWD, 2019. Jahresniederschlag und Jahrestemperatur der Wetterstation Hamburg-Fuhlsbüttel.
Ervin, G. N. & R. G. Wetzel, 2001. Seed fall and field germination of needlerush, Juncus effusus L. Aquatic Botany 71: 233–237.
Fan, Y., H. Li & G. Miguez-Macho, 2013. Global patterns of groundwater table depth. Science 339: 940–943.
FLL, 2016. Regel-Saatgut-Mischungen Rasen (RSM Rasen). Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau e.V, Bonn.
Gallagher, M. T., J. W. Snodgrass, A. B. Brand, R. E. Casey, S. M. Lev & R. J. Van Meter, 2014. The role of pollutant accumulation in determining the use of stormwater ponds by amphibians. Wetlands Ecology and Management 22: 551–564.
Grime, J. P., 1979. Plant strategies and vegetation processes. Wiley, Chichester.
Hamer, A. J., P. J. Smith & M. J. McDonnell, 2012. The importance of habitat design and aquatic connectivity in amphibian use of urban stormwater retention ponds. Urban Ecosystems 15: 451–471.
Hanf, M., 1990. Ackerunkräuter Europas mit ihren Keimlingen und Samen. BVL Verlagsgesellschaft, München.
Hassall, C. & S. Anderson, 2015. Stormwater ponds can contain comparable biodiversity to unmanaged wetlands in urban areas. Hydrobiologia 745: 137–149.
Henry, C. P., C. Amoros & G. Bornette, 1996. Species traits and recolonization processes after flood disturbances in riverine macrophytes. Vegetatio 122: 13–27.
Holtmann, L., K. Philipp, C. Becke & T. Fartmann, 2017. Effects of habitat and landscape quality on amphibian assemblages of urban stormwater ponds. Urban Ecosystems 20: 1249–1259.
Holtmann, L., M. Juchem, J. Brüggeshemke, A. Möhlmeyer & T. Fartmann, 2018. Stormwater ponds promote dragonfly (Odonata) species richness and density in urban areas. Ecological Engineering 118: 1–11.
Holtmann, L., K. Kerler, L. Wolfgart, C. Schmidt & T. Fartmann, 2019. Habitat heterogeneity determines plant species richness in urban stormwater ponds. Ecological Engineering 138: 434–443.
Hopfensperger, K. N., 2007. A review of similarity between seed bank and standing vegetation across ecosystems. Oikos 116: 1438–1448.
Hopfensperger, K. N., K. A. M. Engelhardt & T. R. Lookingbill, 2009. Vegetation and seed bank dynamics in a tidal freshwater marsh. Journal of Vegetation Science 20: 767–778.
Jäger, E. J. (ed.), 2017. Rothmaler Exkursionsflora von Deutschland - Gefäßpflanzen: Grundband. Springer, Heidelberg.
Jensen, K., 1998. Species composition of soil seed bank and seed rain of abandoned wet meadows and their relation to aboveground vegetation. Flora 193: 345–359.
Jensen, K., 2004. Langlebigkeit der Diasporenbanken von Arten der Niedermoorflora Nordwest-Deutschlands: Überblick und Methodenvergleich. Berichte der Reinhold-Tüxen-Gesellschaft 16: 17–28.
Keddy, P. A. & T. H. Ellis, 1985. Seedling recruitment of 11 wetland plant species along a water level gradient: shared or distinct responses? Canadian Journal of Botany 63: 1876–1879.
Kimura, H. & S. Tsuyuzaki, 2011. Fire severity affects vegetation and seed bank in a wetland. Applied Vegetation Science 14: 350–357.
Landesbetrieb Geoinformation und Vermessung, Hamburg, 2020. Digitale Orthophotos (belaubt und unbelaubt) Hamburg. http://suche.transparenz.hamburg.de/dataset/digitale-orthophotos-belaubt-hamburg2?forceWeb=true. http://suche.transparenz.hamburg.de/dataset/digitale-orthophotos-hamburg1?forceWeb=true. Data access: 17.03.2020
Le Viol, I., F. Chiron, R. Julliard & C. Kerbiriou, 2012. More amphibians than expected in highway stormwater ponds. Ecological Engineering 47: 146–154.
Ludewig, K., A. Wanner & K. Jensen, 2015. Recolonization and facilitation in Baltic salt marsh vegetation. Annales Botanici Fennici 52: 181–191.
Ludewig, K., B. Zelle, R. L. Eckstein, E. Mosner, A. Otte & T. W. Donath, 2014. Differential effects of reduced water potential on the germination of floodplain grassland species indicative of wet and dry habitats. Seed Science Research 24: 49–61.
Lundy, L. & R. Wade, 2011. Integrating sciences to sustain urban ecosystem services. Progress in Physical Geography 35: 653–669.
McCune, B. & M. J. Mefford, 2006. PC-ORD. Multivariate analysis of ecological data, MjM Software, Oregon.
Moore, T. L. C. & W. F. Hunt, 2012. Ecosystem service provision by stormwater wetlands and ponds—a means for evaluation? Water Research 46: 6811–6823.
Pickett, S. T. A., M. L. Cadenasso, J. M. Grove, C. H. Nilon, R. V. Pouyat, W. C. Zipperer & R. Costanza, 2001. Urban ecological systems: linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annual Review of Ecology and Systematics 32: 127–157.
Poppendieck, H.-H., H. Bertram, I. Brandt, B. Engelschall & J. von Prondzinski (eds.), 2010. Der Hamburger Pflanzenatlas: von a bis z. Dölling und Galitz, Hamburg.
Raabe, E.-W., 1975. Gramineen-Bestimmungsschlüssel. Kieler Notizen zur Pflanzenkunde 7: 17–44.
Roelofs, J. G. M., E. Brouwer & R. Bobbink, 2002. Restoration of aquatic macrophyte vegetation in acidified and eutrophicated shallow soft water wetlands in the Netherlands. Hydrobiologia 478: 171–180.
Sala, O. E., F. S. Chapin, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sanwald, L. F. Huenneke, R. B. Jackson, A. Kinzig, R. Leemans, D. M. Lodge, H. A. Mooney, M. Oesterheld, N. L. Poff, M. T. Sykes, B. H. Walker, M. Walker & D. H. Wall, 2000. Global biodiversity scenarios for the year 2100. Science 287: 1770–1774.
Scalenghe, R. & F. A. Marsan, 2009. The anthropogenic sealing of soils in urban areas. Landscape and Urban Planning 90: 1–10.
Scher, O. & A. Thièry, 2005. Odonata, Amphibia and environmental characteristics in motorway stormwater retention ponds (Southern France). Hydrobiologia 551: 237–251.
Schlünzen, K. H., P. Hoffmann, G. Rosenhagen & W. Riecke, 2010. Long-term changes and regional differences in temperature and precipitation in the metropolitan area of Hamburg. International Journal of Climatology 30: 1121–1136.
Schütz, W., 2000. Ecology of seed dormancy and germination in sedges (Carex). Perspectives in Plant Ecology, Evolution and Systematics 3: 67–89.
Smith, J. A., A. J. Miller, M. L. Baeck, P. A. Nelson, G. T. Fisher & K. L. Meierdiercks, 2005. Extraordinary flood response of a small urban watershed to short-duration convective rainfall. Journal of Hydrometeorology 6: 599–617.
Solga, A., 2001. Regenrückhaltebecken - Verkannte Lebensräume seltener und gefährdeter Moosarten. Natur und Landschaft 76: 23–25.
Stahlschmidt, P., A. Pätzold, L. Ressl, R. Schulz & C. A. Brühl, 2012. Constructed wetlands support bats in agricultural landscapes. Basic and Applied Ecology 13: 196–203.
Statistische Ämter des Bundes und der Länder, 2018. Flächennutzung Hamburg. Data access: 07.01.2019.
StatSoft Inc., 2014. STATISTICA für Windows (Software-Systeme für Datenanalyse). Tulsa.
Ter Heerdt, G. N. J., G. L. Verweij, R. M. Bekker & J. P. Bakker, 1996. An improved method for seed-bank analysis: seedling emergence after removing the soil by sieving. Functional Ecology 10: 144–151.
Thompson, K., J. Bakker & R. Bekker, 1997. The soil seed banks of North West Europe: methodology, density and longevity. University Press, Cambridge.
Vogt, K., L. Rasran & K. Jensen, 2004. Water-borne seed transport and seed deposition during flooding in a small river-valley in Northern Germany. Flora 199: 377–388.
Acknowledgements
Open Access funding provided by Projekt DEAL. We thank Rudolf Hennemann from the planning office Plan.et and Stephan Schneider from the administrative district Eimsbüttel (Hamburg) for background information and the latter also for permission to work in the pond. We further thank Imke Bodendieck, Christian Pawlak and Thea Wahlers for field and lab assistance. We thank three anonymous reviewers for valuable comments on our manuscript. Nikola Lenzewski was funded by the Federal Ministry of Education and Research (Germany) within the framework of the StucK-Project (033W031B). Kristin Ludewig was financed within the MediAN-Project (01LC1601A) by the Federal Ministry of Education and Research (Germany).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Franziska Eller, Hans Brix, Brian K. Sorrell & Carlos A. Arias / Wetland ecosystems: functions and use in a changing climate.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lenzewski, N., Jensen, K. & Ludewig, K. Seed bank has the potential for re-colonising urban stormwater ponds after reconstruction. Hydrobiologia 848, 3305–3316 (2021). https://doi.org/10.1007/s10750-020-04365-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04365-0