Abstract
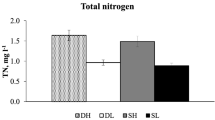
Microbialites are microbial communities that create a carbonate structure. They are abundant in the Great Salt Lake, a hypersaline lake in the arid Great Basin of the USA, where they contribute to overall primary production, seasonally up to 55%. While the microbial diversity of microbialites has been investigated, how abiotic factors affect the abundance of their primary constituents is not well understood. We examined how microbialite primary producers respond to varying levels of temperature, salinity, and nitrogen within ranges observed in the Great Salt Lake. All abiotic factors and their interactions significantly affected the maximum chlorophyll-a abundance, suggesting that these factors co-limit microbialite primary producers in the Great Salt Lake. Maximum chlorophyll-a concentrations increased with nitrogen additions and showed a parabolic relationship with salinity and temperature with peaks around 60 ppt and 20°C, respectively. While salinity had a strong effect on microbialite primary producers, we found that temperature and nitrogen were more impactful, accounting for 40 and 30% of the variance in maximum abundance, respectively, while salinity contributed just 15%. Our results show the importance of the interplay of abiotic factors on Great Salt Lake microbialites and highlight the need for increased study of benthic communities in inland saline lakes.



Similar content being viewed by others
Data availability
Data is available by contacting the corresponding author (kbailey9@nd.edu).
References
Alcorlo, P., A. Baltanas & C. Montes, 2001. Food-web structure in two shallow salt lakes in Los Monegros (NE Spain): energetic vs dynamic constraints. Hydrobiologia 466: 307–316.
Arar, E. J. & G. B. Collins, 1997. Method 445.0: In Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorescence. U.S. Environmental Protection Agency, Washington, DC.
Barrett, K. L. & G. E. Belovsky, 2020. Invertebrates and phytoplankton: is salinity the driving factor? In Baxter, B. K. & J. K. Butler (eds), Great Salt Lake Biology: A Terminal Lake in a Time of Change. Springer, Dordrecht.
Baskin, R. L., 2014. Occurrence and Spatial Distribution of Microbial Bioherms in Great Salt Lake, Utah. The University of Utah, Utah.
Baulch, H. M., D. W. Schindler & M. A. Turner, 2005. Effects of warming on benthic communities in a boreal lake: implications of climate change. Limnology and Oceanography 50: 1377–1392.
Belovsky, G. E., D. Stephens, C. Perschon, P. Birdsey, D. Paul, D. Naftz, R. Baskin, C. Larson, C. Mellison, J. Luft, R. Mosley, H. Mahon, J. Van Leeuwen & D. V. Allen, 2011. The Great Salt Lake Ecosystem (Utah, USA): long term data and a structural equation approach. Ecosphere 2: 1–40.
Camacho, A. & R. de Wit, 2003. Effect of nitrogen and phosphorus additions on a benthic microbial mat from a hypersaline lake. Aquatic Microbial Ecology 32: 261–273.
Cao, Y., S. Olsen, M. Florencia Gutierrez, S. Brucet, T. A. Davidson, W. Li, T. L. Lauridsen, M. Sondergaard & E. Jeppesen, 2017. Temperature effects on periphyton, epiphyton and epipelon under a nitrogen pulse in low-nutrient experimental freshwater lakes. Hydrobiologia 795: 267–279.
Carozzi, A. V., 1962. Observations on algal biostromes in the Great Salt Lake, Utah. The Journal of Geology 70: 246–252.
Caumette, P., R. Matheron, N. Raymond & J.-C. Relexans, 1994. Microbial mats in the hypersaline ponds of Mediterranean salterns (Salins-de-Giraud, France). FEMS Microbiology Ecology 13: 273–286.
Centeno, C. M., O. Mejía & L. I. Falcón, 2016. Habitat conditions drive phylogenetic structure of dominant bacterial phyla of microbialite communities from different locations in Mexico. Revista de Biologia Tropical 64: 1057–1065.
Chiu, J. M. Y., V. Thiyagarajan, M. M. Y. Tsoi & P. Y. Qian, 2005. Qualitative and quantitative changes in marine biofilms as a function of temperature and salinity in summer and winter. Biofilms 2: 183–195.
Collins, N., 1980. Population ecology of Ephydra cinerea Jones (Diptera: Ephydridae), the only benthic metazoan of the Great Salt Lake, USA. Hydrobiologia 68: 99–112.
Cook, B. I., T. R. Ault & J. E. Smerdon, 2015. Unprecedented 21st century drought risk in the American Southwest and Central Plains. Science Advances 1: e1400082.
Diaz, P., M. C. Guerrero, P. Alcorlo, A. Baltanas, M. Florin & C. Montes, 1998. Anthropogenic perturbations to the trophic structure in a permanent hypersaline shallow lake: La Salada de Chiprana (north-eastern Spain). International Journal of Salt Lake Research 7: 187–210.
Edgcomb, V. P., J. M. Bernhard, R. E. Summons, W. Orsi, D. Beaudoin & P. T. Visscher, 2014. Active eukaryotes in microbialites from Highborne Cay, Bahamas, and Hamelin Pool (Shark Bay), Australia. The ISME Journal 8: 418–429.
Edwards, E. C. & S. E. Null, 2019. The cost of addressing saline lake level decline and the potential for water conservation markets. Science of the Total Environment 651: 435–442.
Frank, M. G. & M. R. Conover, 2019. Threatened Habitat at Great Salt Lake: Importance of Shallow-Water and Brackish Habitats to Wilson’s and Red-Necked Phalaropes. The Condor Oxford University Press, Oxford.
Guerrero, M. C. & R. de Wit, 1992. Microbial mats in the inland saline lakes of Spain. Limnetica 8: 197–204.
Havemann, S. A. & J. S. Foster, 2008. Comparative characterization of the microbial diversities of an artificial microbialite model and a natural stromatolite. Applied and Environmental Microbiology 74: 7410–7421.
Havens, K. & E. Jeppesen, 2018. Ecological responses of lakes to climate change. WATER 10: 917.
Herbst, D. B. & D. W. Blinn, 1998. Experimental mesocosm studies of salinity effects on the benthic algal community of a saline lake. Journal of Phycology 34: 772–778.
Herbst, D. B. & T. J. Bradley, 1989. Salinity and nutrient limitations on growth of benthic algae from two alkaline salt lakes of the western Great Basin (USA). Journal of Phycology 25: 673–678.
Hong, H. P. & J. K. Choi, 2015. Can the halophilic ciliate Fabrea salina be used as a bio-control of microalgae blooms in solar salterns? Ocean Science Journal 50: 529–536.
Kirchman, D. L., 2016. Growth rates of microbes in the oceans. Annual Review of Marine Science 8: 285–309.
Larson, C. A. & G. E. Belovsky, 2013. Salinity and nutrients influence species richness and evenness of phytoplankton communities in microcosm experiments from Great Salt Lake, Utah, USA. Journal of Plankton Research 35: 1154–1166.
Lee, K. H., H. J. Jeong, K. Lee, P. J. S. Franks, K. A. Seong, S. Y. Lee, M. J. Lee, S. Hyeon Jang, E. Potvin, A. Suk Lim, E. Y. Yoon, Y. D. Yoo, N. S. Kang & K. Y. Kim, 2019. Effects of warming and eutrophication on coastal phytoplankton production. Harmful Algae 81: 106–118.
Li, W., X. Xu, M. Fujibayashi, Q. Niu, N. Tanaka & O. Nishimura, 2016. Response of microalgae to elevated CO2 and temperature: impact of climate change on freshwater ecosystems. Environmental Science and Pollution Research International 23: 19847–19860.
Lind, L., M. S. Schuler, W. D. Hintz, A. B. Stoler, D. K. Jones, B. M. Mattes & R. A. Relyea, 2018. Salty fertile lakes: how salinization and eutrophication alter the structure of freshwater communities. Ecosphere 9: 1–19.
Lindsay, M. R., C. Anderson, N. Fox, G. Scofield, J. Allen, E. Anderson, L. Bueter, S. Poudel, K. Sutherland, J. H. Munson-McGee, J. D. Van Nostrand, J. Zhou, J. R. Spear, B. K. Baxter, D. R. Lageson & E. S. Boyd, 2017. Microbialite response to an anthropogenic salinity gradient in Great Salt Lake, Utah. Geobiology 15: 131–145.
Lindsay, M. R., R. E. Johnston, B. K. Baxter & E. S. Boyd, 2019. Effects of salinity on microbialite-associated production in Great Salt Lake, Utah. Ecology 100: e02611.
Naftz, D., 2017. Inputs and internal cycling of nitrogen to a causeway influenced, hypersaline lake, Great Salt Lake, Utah, USA. Aquatic Geochemistry 23: 199–216.
Norf, H. & M. Weitere, 2010. Resource quantity and seasonal background alter warming effects on communities of biofilm ciliates. FEMS Microbiology Ecology 74: 361–370.
Pei, G.-F., G.-X. Liu & Z.-Y. Hu, 2010. A comparative study of benthic algal colonization in shallow lakes of China. Journal of Freshwater Ecology 25: 403–411.
Pinckney, J., H. W. Paerl & B. M. Bebout, 1995. Salinity control of benthic microbial mat community production in a Bahamian hypersaline lagoon. Journal of Experimental Marine Biology and Ecology 187: 223–237.
Prieto-Barajas, C. M., E. Valencia-Cantero & G. Santoyo, 2018. Microbial mat ecosystems: structure types, functional diversity, and biotechnological application. Electronic Journal of Biotechnology 31: 48–56.
R Core Team, 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Roberts, A. J. & M. R. Conover, 2014. Role of benthic substrate in waterbird distribution on Great Salt Lake, Utah. Waterbirds 37: 298–306.
Roberts, A. J., M. R. Conover, J. Luft & J. Neill, 2013. Population fluctuations and distribution of staging Eared Grebes (Podiceps nigricollis) in North America. Canadian Journal of Zoology 91: 906–913.
Ruvindy, R., R. A. White 3rd, B. A. Neilan & B. P. Burns, 2016. Unravelling core microbial metabolisms in the hypersaline microbial mats of Shark Bay using high-throughput metagenomics. The ISME Journal 10: 183–196.
Scholz, B. & G. Liebezeit, 2012. Growth responses of 25 benthic marine Wadden Sea diatoms isolated from the Solthörn tidal flat (southern North Sea) in relation to varying culture conditions. Diatom Research 27: 65–73.
Shadrin, N. V., 2018. The alternative saline lake ecosystem states and adaptive environmental management. Journal of Oceanology and Limnology 36: 2010–2017.
Smith, M. D., S. E. Goater, E. S. Reichwaldt, B. Knott & A. Ghadouani, 2010. Effects of recent increases in salinity and nutrient concentrations on the microbialite community of Lake Clifton (Western Australia): are the thrombolites at risk? Hydrobiologia 649: 207–216.
Stenger-Kovacs, C., E. Lengyel, K. Buczko, F. M. Toth, L. O. Crossetti, A. Pellinger, Z. Z. Doma & J. Padisak, 2014. Vanishing world: alkaline, saline lakes in Central Europe and their diatom assemblages. Inland Waters 4: 383–396.
Stephens, D. W., 1990. Changes in lake levels, salinity and the biological community of Great Salt Lake (Utah, USA), 1847–1987. Hydrobiologia 197: 139–146.
Stephens, D. W., 1998. Salinity-Induced Changes in the Aquatic Ecosystem of Great Salt Lake, Utah Utah Geological Association Guidebook 26. U.S. Geological Survey, Salt Lake City.
Stephens, D. W. & D. M. Gillespie, 1976. Phytoplankton production in Great Salt Lake, Utah, and a laboratory study of algal response to enrichment. Limnology and Oceanography 21: 74–87.
Stolz, J. F., 1990. Distribution of phototrophic microbes in the flat laminated microbial mat at Laguna Figueroa, Baja California, Mexico. BioSystems 23: 345–357.
Vadeboncoeur, Y. & M. E. Power, 2017. Attached algae: the cryptic base of inverted trophic pyramids in freshwaters. Annual Review of Ecology, Evolution, and Systematics 48: 255–279.
Vadeboncoeur, Y., M. J. Vander Zanden & D. M. Lodge, 2002. Putting the lake back together: reintegrating benthic pathways into lake food web models. AIBS Bulletin 52: 44–54.
Vadeboncoeur, Y., E. Jeppesen, M. J. V. Zanden, H.-H. Schierup, K. Christoffersen & D. M. Lodge, 2003. From Greenland to green lakes: cultural eutrophication and the loss of benthic pathways in lakes. Limnology and Oceanography 48: 1408–1418.
van der Grinten, E., P. H. Arni, K. de Mutsert, C. Barranguet & W. Admiraal, 2005. Temperature- and light-dependent performance of the cyanobacterium Leptolyngbya foveolarum and the diatom Nitzschia perminuta in mixed biofilms. Hydrobiologia 548: 267–278.
Villanueva, V. D., J. Font, T. Schwartz & A. M. Romani, 2011. Biofilm formation at warming temperature: acceleration of microbial colonization and microbial interactive effects. Biofouling 27: 59–71.
Wieland, A. & M. Kühl, 2006. Regulation of photosynthesis and oxygen consumption in a hypersaline cyanobacterial mat (Camargue, France) by irradiance, temperature and salinity. FEMS Microbiology Ecology 55: 195–210.
Williams, W. D., 2002. Environmental threats to salt lakes and the likely status of inland saline ecosystems in 2025. Environmental Conservation 29: 154–167.
Williams, W. D., A. J. Boulton & R. G. Taaffe, 1990. Salinity as a determinant of salt lake fauna: a question of scale. Hydrobiologia 197: 257–266.
Wurtsbaugh, W. A., 1992. Food-web modification by an invertebrate predator in the Great Salt Lake (USA). Oecologia 89: 168–175.
Wurtsbaugh, W. A., 2009. Biostromes, brine flies, birds and the bioaccumulation of selenium in Great Salt Lake, Utah. Natural Resources and Environmental Issues 15: 1–13.
Wurtsbaugh, W. A., 2018. Effects of eutrophication on birds in three bays of Great Salt Lake: A comparative analysis with Utah DWR Waterbird Survey Data. Utah Division of Forestry, Fire and State Lands, Cedar City.
Wurtsbaugh, W. A., J. Gardberg & C. Izdepski, 2011. Biostrome communities and mercury and selenium bioaccumulation in the Great Salt Lake (Utah, USA). The Science of the Total Environment 409: 4425–4434.
Wurtsbaugh, W., C. Miller, S. Null, P. Wilcock, M. Hahnenberger & F. Howe, 2016. Impacts of water development on Great Salt Lake and the Wasatch Front. Utah State University, Logan.
Acknowledgements
Field assistance and funding were provided by the Great Salt Lake Ecosystem Program, Utah Division of Wildlife Resources. M. Igleski and L. Herrera aided in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling editor: Stefano Amalfitano
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anderson, N.L., Barrett, K.L., Jones, S.E. et al. Impact of abiotic factors on microbialite growth (Great Salt Lake, Utah, USA): a tank experiment. Hydrobiologia 847, 2113–2122 (2020). https://doi.org/10.1007/s10750-020-04235-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04235-9