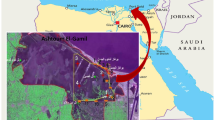
Abstract
Neotropical freshwater ecosystems are experiencing a great expansion in the number of invasive species, which is especially alarming since this region harbours 30% of the world’s fish biodiversity with high levels of endemism. We aimed to evaluate the main predictors of peacock basses (Cichla spp.) abundance outside their native range, which are the Amazon and Tocantins-Araguaia river basins. We used multivariate ordination techniques and multimodel inference to analyse peacock basses abundance in twelve reservoirs of the Paraíba do Sul river basin, southeastern Brazil. Interestingly, reservoirs at higher (southernmost) latitudes, located in more populated areas, had higher water temperature and lower turbidity, due to increased water residence time, and these three variables were also positively correlated with abundance of this warm-water invasive fish. Habitat structure was less important in explaining peacock basses abundance, which was not significantly related to biotic factors (fish species richness and time since peacock basses introduction). We hypothesize that the observed effects of reservoir management on limnological features and peacock bass abundance, particularly water residence time (as a mediator of temperature and turbidity), may apply to other Neotropical basins and could influence the impact of this invader.





Similar content being viewed by others
References
Agostinho, A. A., L. E. Miranda, L. M. Bini, L. C. Gomes, S. M. Thomaz & H. I. Suzuki, 1999. Patterns of Colonization in Neotropical Reservoirs, and Prognosis on Aging. In Tundisi, J. G. & M. Straškraba (eds), Theoretical Reservoir Ecology and its Applications. International Institute of Ecology, São Carlos: 227–265.
Agostinho, A. A., L. C. Gomes & F. M. Pelicice, 2007. Ecologia e manejo de recursos pesqueiros em reservatórios do Brasil. Eduem, Maringá.
Bailly, D., F. A. S. Cassemiro, K. O. Winemiller, J. A. F. Diniz-Filho & A. A. Agostinho, 2016. Diversity gradients of Neotropical freshwater fish: evidence of multiple underlying factors in human-modified systems. Journal of Biogeography 43: 1679–1689.
Bartón, K., 2016. Package “MuMIn” version 1.15.6. https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf
Blanchet, G., P. Legendre & D. Borcard, 2008. Forward selection of spatial explanatory variables. Ecology 89: 2623–2632.
Borcard, D., P. Legendre & P. Drapeau, 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045–1055.
Borcard, D., F. Gillet & P. Legendre, 2011. Numerical Ecology with R. Springer, New York: 319.
Brook, B. W., N. S. Sodhi & C. J. A. Bradshaw, 2008. Synergies among extinction drivers under global change. Trends in Ecology and Evolution 23: 453–460.
Bulleri, F., J. F. Bruno & L. Benedetti-Cecchi, 2008. Beyond competition: incorporating positive interactions between species to predict ecosystem invasibility. PLoS Biology 6: e162.
Burnham, K. P. & D. R. Anderson, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer, New York.
Byers, J. E., 2002. Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes. Oikos 97: 449–458.
Carmona-Catot, G., A. F. G. N. Santos, P. A. Tedesco & E. García-Berthou, 2014. Quantifying seasonality along a latitudinal gradient: from stream temperature to growth of invasive mosquitofish. Ecosphere 5(10): 1–23.
Carol, J., L. Benejam, J. Benito & E. García-Berthou, 2009. Growth and diet of European catfish (Silurus glanis) in early and late invasion stages. Fundamental and Applied Limnology 174: 317–328.
Cavalcanti, B. S. & G. G. Marques, 2016. Recursos hídricos e gestão de conflitos: a bacia hidrográfica do rio Paraíba do Sul a partir da crise hídrica de 2014–2015. Revista Portuguesa e Brasileira de Gestão 15: 4–16.
CEIVAP, 2017. Comitê de Integração da Bacia Hidrográfica do Rio Paraíba do Sul. http://www.ceivap.org.br/index.php
Clavero, M. & E. García-Berthou, 2005. Invasive species are a leading cause of animal extinctions. Trends in Ecology & Evolution 20: 110–110.
Colautti, R. I., I. A. Grigorovich & H. J. MacIsaac, 2006. Propagule pressure: a null model for biological invasions. Biological Invasions 8: 1023–1037.
Cucherousset, J. & J. D. Olden, 2011. Ecological impacts of nonnative freshwater fishes. Fisheries 36: 215–230.
Dray, S., P. Legendre, & G. Blanchet, 2016. Packfor: Forward Selection with permutation (Canoco p.46). R package version 0.0-8/r136. https://R-Forge.R-project.org/projects/sedar/
Espínola, L. A., C. V. Minte-Vera & H. F. Júlio, 2010. Invasibility of reservoirs in the Paraná Basin, Brazil, to Cichla kelberi Kullander and Ferreira, 2006. Biological Invasions 12: 1873–1888.
Espínola, L. A., C. V. Minte-Vera, H. F. Júnio-Junior, L. N. Santos & K. O. Winemiller, 2015. Evaluation of factors associated with dynamics of Cichla ocellaris invasion of the Upper Parana River floodplain system, Brazil. Marine and Freshwater Research 66: 33–40.
Gido, K. B., W. J. Matthews & W. C. Wolfinbarger, 2000. Long-term changes in a reservoir fish assemblage: stability in an unpredictable environment. Ecological Applications 10: 1517–1529.
Gomes, J. H. C., A. C. I. M. Dias & C. W. C. W. C. Branco, 2008. Fish assemblage composition in three reservoirs in the State of Rio de Janeiro. Acta Limnologica Brasiliensia 20: 117–130.
Havel, J. E., C. E. Lee & M. J. Vander Zanden, 2005. Do reservoirs facilitate invasions into landscapes. BioScience 55: 518–525.
Hawkins, C. P., J. N. Hogue, L. M. Decker & J. W. Feminella, 1997. Channel morphology, water temperature, and assemblage structure of stream insects. Journal of the North American Benthological Society 16: 728–749.
Hickley, P., M. Muchiri, R. Britton & R. Boar, 2008. Economic gain versus ecological damage from the introduction of non-native freshwater fish: case studies from Kenya. The Open Fish Science Journal 1: 36–46.
Hoeinghaus, D. J., C. A. Layman, D. A. Arrington & K. O. Winemiller, 2003. Movement of Cichla species (Cichlidae) in a Venezuelan floodplain river. Neotropical Ichthyology 1: 121–126.
Irz, P., A. Laurent, S. Messad, O. Pronier & C. Argillier, 2002. Influence of site characteristics on fish community patterns in French reservoirs. Ecology of Freshwater Fish 11: 123–136.
Jackson, D. A., 1993. Stopping rules in principal component analysis: a comparison of heuristical and statistical approaches. Ecology 74: 2204–2214.
Johnson, P. T. J., J. D. Olden & M. J. Vander Zanden, 2008. Dam invaders: impoundments facilitate biological invasions into freshwaters. Frontiers in Ecology and the Environment 6: 357–363.
Kennedy, R. H. & W. W. Walker, 1990. Reservoir Nutrient Dynamics. In Thornton, K. W., B. L. Kimmel & F. E. Payne (eds), Reservoir Limnology: Ecological Perspectives. Wiley-Interscience, New York: 109–132.
Kindt, R., 2017. Package “BiodiversityR” for community ecology and suitability analysis. R package version 2.8-0. https://cran.r-project.org/web/packages/BiodiversityR/BiodiversityR.pdf
Kovalenko, K. E., E. D. Dibble, A. A. Agostinho, G. Cantanhêde & R. Fugi, 2010. Direct and indirect effects of an introduced piscivore, Cichla kelberi and their modification by aquatic plants. Hydrobiologia 638: 245–253.
Kullander, S. O. & E. J. G. Ferreira, 2006. A review of South American cichlid genus Cichla, with descriptions of nine new species (Teleostei: Cichlidae). Ichthyological Exploration of Freshwaters 17: 289–398.
Latini, A. O. & M. Petrere, 2004. Reduction of a native fish fauna by alien species: an example from Brazilian freshwater tropical lakes. Fisheries Management and Ecology 11: 71–79.
Legendre, P. & L. Legendre, 2012. Numerical Ecology, 3rd ed. Elsevier, Amsterdam.
Levine, J. M. & C. M. D’Antonio, 1999. Elton revisited: a review of evidence linking diversity and invasibility. Oikos 87: 15.
Levine, J. M., P. B. Adler & S. G. Yelenik, 2004. A meta-analysis of biotic resistance to exotic plants invasions. Ecology Letters 7: 975–989.
Liew, J. H., H. H. Tan & D. C. J. Yeo, 2016. Dammed rivers: impoundments facilitate fish invasions. Freshwater Biology 61: 1421–1429.
Lowe-McConnell, R. H., 1969. The cichlid fishes of Guyana, South America, with notes on their ecology and breeding behavior. Zoological Journal of the Linnean Society 48: 255–302.
Mack, R. N., D. Simberloff, W. Mark Lonsdale, H. Evans, M. Clout & F. A. Bazzaz, 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications 10: 689–710.
Marengo, J.A. & L.M. Alves, 2005. Tendências Hidrológicas da Bacia do Rio Paraíba do Sul. INPE e-print. http://mtc-m16c.sid.inpe.br/col/sid.inpe.br/ePrint@80/2005/05.11.13.21/doc/v1.pdf
Marques, A. C. P. B., A. C. S. Franco, F. Salgueiro, E. García-Berthou & L. N. Santos, 2016. Genetic divergence among invasive and native populations of the yellow peacock cichlid Cichla kelberi. Journal of Fish Biology 89: 2595–2606.
Oksanen, J., F.G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P.R. Minchin, R.B. O’Hara, G.L. Simpson, P. Solymos, M. Henry, H. Stevens, E. Szoecs & H. Wagner, 2016. vegan: Community ecology package. R package version 2.4-1. https://CRAN.R-project.org/package=vegan
Pelicice, F. M., J. D. Latini & A. A. Agostinho, 2015. Fish fauna disassembly after the introduction of a voracious predator: main drivers and the role of the invader’s demography. Hydrobiologia 746: 271–283.
Peña, E. A. & E. H. Slate, 2006. Global validation of linear model assumptions. Journal of the American Statistical Association 101: 341–354.
Pinto, B.C.T., 2008. Condicionantes ambientais estruturadoras das assembléias de peixes da bacia do rio Paraíba do Sul: condição do uso da terra, do habitat físico e qualidade físico-química da água. PhD. Thesis in Animal Biology, PPGBA, Universidade Federal Rural do Rio de Janeiro, Seropédica, 200 pp.
Quist, M. C., F. J. Rahel & W. A. Hubert, 2005. Hierarchical faunal filters: an approach to assessing effects of habitat and nonnative species on native fishes. Ecology of Freshwater Fish 14: 24–39.
R Core Team, 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org/
Richardson, W. B. & L. A. Bartsch, 1997. Effects of zebra mussels on food webs: interactions with juvenile bluegill and water residence time. Hydrobiologia 354: 141–150.
Rueda, F., E. Moreno-Ostos & J. Armengol, 2006. The residence time of river water in reservoirs. Ecological Modelling 191: 260–274.
Santos, L. N., A. F. Gonzales & F. G. Araújo, 2001. Dieta do tucunaré-amarelo Cichla monoculus (Bloch & Schneider) (Osteichthyes, Cichlidae), no reservatório de Lajes, Rio de Janeiro, Brasil. Revista Brasileira de Zoologia 18: 191–204.
Santos, L. N., E. García-Berthou, A. A. Agostinho & J. D. Latini, 2011. Fish colonization of artificial reefs in a large Neotropical reservoir: material type and successional changes. Ecological Applications 21: 251–262.
Santos, L. N., A. C. S. Franco, A. Marques, F. Nóbrega & F. Salgueiro, 2016a. Molecular analysis confirms the introduction of a second species of yellow peacock cichlid Cichla monoculus Spix & Agassiz 1831 (Cichliformes: Cichlidae) in the Southeast Atlantic Hydrographic province, Brazil. BioInvasions Records 5: 277–284.
Santos, L. N., F. Salgueiro, A. C. S. Franco, A. C. P. Marques & F. Nóbrega, 2016b. First record of the invasive blue peacock cichlid Cichla piquiti Kullander and Ferreira 2006 (Cichliformes: Cichlidae) in the Paraíba do Sul river basin, south eastern Brazil. BioInvasions Records 5: 267–275.
Shafland, P. L., 1996. Reviews in fisheries science exotic fishes of Florida—1994. Reviews in Fisheries Science 4: 101–122.
Sharpe, D. M. T., L. F. De León, R. González & M. E. Torchin, 2017. Tropical fish community does not recover 45 years after predator introduction. Ecology 98: 412–424.
Soballe, D. M. & B. L. Kimmel, 1987. A large-scale comparison of factors influencing phytoplankton abundance in rivers, lakes, and impoundments. Ecology 68: 1943–1954.
Straškraba, M., 1999. Retention Time as a Key Variable of Reservoir Limnology. In Tundisi, J.G. & M. Straškraba (eds), Theoretical Reservoir Ecology and its Applications. São Carlos: International Institute of Ecology, Brazilian Academy of Sciences, Backhuys Publishers: 385–410.
Straškraba, M., J. G. Tundisi, & A. Duncan, 1993. In Comparative Reservoir Limnology and Water Quality Management. Springer, Netherlands: 213–288.
Thomaz, S. M., A. A. Agostinho, L. C. Gomes, M. J. Silveira, M. Rejmánek, C. E. Aslan & E. Chow, 2012. Using space-for-time substitution and time sequence approaches in invasion ecology. Freshwater Biology 57: 2401–2410.
Willis, S. C., J. Macrander, I. P. Farias & G. Ortí, 2012. Simultaneous delimitation of species and quantification of interspecific hybridization in Amazonian peacock cichlids (genus Cichla) using multi-locus data. BMC Evolutionary Biology 12: 96.
Winemiller, K. O., 2001. Ecology of peacock cichlids (Cichla spp.) in Venezuela. Journal of Aquariculture and Aquatic Sciences 9: 93–112.
Winemiller, K. O., D. C. Taphorn & A. Barbarino-Duque, 1997. Ecology of Cichla (Cichlidae) in two blackwater rivers of southern Venezuela. Copeia 1997: 690–696.
Yeo, D. C. J. & C. S. W. Chia, 2010. Introduced species in Singapore: an overview. Cosmos 6: 23–37.
Zaret, T. M. & R. T. Paine, 1973. Species introduction in a tropical lake. Science 182: 449–455.
Acknowledgements
We thank the Graduate Courses in Ecology (PPGE-UFRJ) and Neotropical Biodiversity (PPGBIO-UNIRIO). We also thank people at Laboratório de Ictiologia Teórica e Aplicada for providing logistic support and two anonymous reviewers for helpful comments that greatly improved the manuscript. This work was funded by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Research Grant to LNS, E-112.644/2012, E-26/202.840/2015), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Research Grant to LNS, ref. 312194/2015-3), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (sandwich doctorate scholarship to ACSF, ref. 88887.127440/2016-00 and visiting professorship to EGB, ref. 88881.068352/2014-01). EGB was also supported by the Spanish Ministry of Economy and Competitiveness (Projects and CGL2015-69311-REDT, CGL2016-80820-R and PCIN-2016-168) and the Government of Catalonia (ref. 2014 SGR 484).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: John E. Havel, Sidinei M. Thomaz, Lee B. Kats, Katya E. Kovalenko & Luciano N. Santos / Aquatic Invasive Species II
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Franco, A.C.S., dos Santos, L.N., Petry, A.C. et al. Abundance of invasive peacock bass increases with water residence time of reservoirs in southeastern Brazil. Hydrobiologia 817, 155–166 (2018). https://doi.org/10.1007/s10750-017-3467-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3467-x