Abstract
Environmental gradients and competition influence aquatic macrophyte distribution in estuaries. The competition-to-stress hypothesis states that some species are excluded from lower estuaries (high salinity) due to abiotic stress and others from upper estuaries (low salinity) by competition. The growth of Crinum americanum L. and Spartina alterniflora Loisel. in monoculture (10:0/0:10) and mixed culture (5:5) under different salinity levels (4/12/26) was analysed by a laboratory experiment (3 cultures × 3 sediment types × 3 replicate) to understand the role of competition and salinity on the distribution of these species in a tropical estuary as well as to verify whether the competition-to-stress hypothesis explains their zonation. We tested the hypothesis that S. alterniflora is not established in the upper estuary due to the effect of competition with C. americanum, whereas the latter presents restrictions to high salinity and has greater competitive ability in the upper estuary. Our data confirm the competition-to-stress hypothesis but not as proposed originally. We conclude that abiotic stress (low nutrient availability) is responsible for the absence of S. alterniflora in the upper estuary and that the competition between the two species is responsible for the absence of C. americanum in the lower estuary.





Similar content being viewed by others
References
Barbour, M. G., 1978. The effect of competition and salinity at growth of a salt marsh species. Oecologia 37: 93–99.
Bertness, M. D., 1991. Zonation of Spartina patens and Spartina alterniflora in a New England salt marsh. Ecology 72: 138–148.
Bertness, M. D., L. Gough & S. W. Shumway, 1992. Salt tolerances and the distribution of fugitive salt marsh plants. Ecology 73: 1842–1851.
Biudes, J. F. V. & A. F. M. Camargo, 2006. Changes in biomass, chemical composition and nutritive value of Spartina alterniflora due to organic pollution in the Itanhaém River Basin (SP, Brazil). Brazilian Journal of Biology 66: 781–789.
Burgos-León, A. M., D. Valdés, M. E. Vega & O. Defeo, 2013. Spatial struturing of submerged aquatic vegetation in an estuarine habitat of the Gulf of Mexico. Journal of the Marine Biological Association of the United Kingdom 93: 855–866.
Camargo, A. F. M. & E. R. Florentino, 2000. Population dynamics and net primary production of the aquatic macrophytes Nymphaea rudgeana C.F. Mey in a lotic environment of the Itanhaém River Basin (SP, Brazil). Brazilian Journal of Biology 60: 83–92.
Camargo, A. F. M., L. A. Pereira & A. M. M. Pereira, 2002. Ecologia da bacia hidrográfica do rio Itanhaém. In Schiavetti, A. & A. F. M. Camargo (eds), Conceitos de Bacias Hidrográficas. Editus, Ilhéus.
Castillo, J. M., A. E. Rubio-Casal, S. Redondo, A. A. Ivarez-Lopez, T. Luque, C. Luque, F. J. Nieva, E. M. Castellanos & M. E. Figueroa, 2005. Short-term responses to salinity of an invasive cordgrass. Biological Invasions 7: 29–35.
Céccoli, G., J. Ramos, V. Pilatti, I. Dellaferrera, J. C. Tivano, E. Taleisnik & A. C. Vegetti, 2015. Salt glands in the Poaceae family and their relationship to salinity tolerance. The Botanical Review 81: 162–178.
Crain, C. M., B. R. Silliman, S. L. Bertness & M. D. Bertness, 2004. Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology 85: 2539–2549.
Darby, F. A. & R. E. Turner, 2008. Below- and aboveground biomass of Spartina alterniflora: response to nutrient addition in a Louisiana Salt Marsh. Estuaries and Coasts 31: 326–334.
Engels, J. G. & K. Jensen, 2010. Role of biotic interactions and physical factors in determining the distribution of marsh species along an estuarine salinity gradient. Oikos 119: 679–685.
Eyre, B. & P. Balls, 1999. A comparative study of nutrient behavior along the salinity gradient of tropical and temperate estuaries. Estuaries 22: 313–326.
French, T. D. & E. P. A. Chambers, 1996. Habitat partitioning in riverine macrophytes communities. Freshwater Biology 36: 509–520.
Golterman, H. L., R. S. Climo & M. A. M. Ohnstad, 1978. Methods for Physical and Chemical Analysis of Freshwaters. IBP, Oxford.
GraphPad Software, 2007. Prism (Data Analysis Software System), Version 5.
Greenwood, M. E. & G. R. MacFarlane, 2009. Effects of salinity on competitive interactions between two juncus species. Aquatic Botany 90: 23–29.
Guo, H. & S. C. Pennings, 2012. Mechanisms mediating plant distributions across estuarine landscapes in a low-latitude tidal estuary. Ecology 93: 90–100.
He, Q. & B. R. Silliman, 2015. Biogeographic consequences of nutrient enrichment for plant–herbivore interactions in coastal wetlands. Ecology Letters 18: 462–471.
Hellquist, C. E. & R. A. Black, 2010. The influence of intertidal zone and native vegetation on the survival and growth of Spartina anglica in Northern Puget Sound, WA, USA. In Ayres, D. R., D. W. Kerr, S. D. Ericson & P. R. Olofson (eds), Proceedings of the Third International Conference on Invasive Spartina, 8–10 November 2004. San Francisco Estuary Invasive Spartina Project of the California State Coastal Conservancy, Oakland, San Francisco, CA.
Henry-Silva, G. G. & A. F. M. Camargo, 2005. Interações ecológicas entre as macrófitas aquáticas flutuantes Eichhornia crassipes e Pistia stratiotes. Hoehnea 32: 445–452.
Kao, J. T., J. E. Titus & W. X. Zhu, 2003. Differential nitrogen and phosphorus retention by five wetland plant species. Wetlands 23: 979–987.
Kenkel, N. C., A. L. Mcllraith, C. A. Burchill & G. Jones, 1991. Competition and the response of three plant species to a salinity gradient. Canadian Journal of Botany 69: 2497–2502.
La Peyre, M. K. G., J. B. Grace, E. Hahn & I. A. Mendelssohn, 2001. The importance of competition in regulating plant species abundance along a salinity gradient. Ecology 82: 62–69.
Lipscshitz, N., A. Shomer-llan, A. Eshel & Y. Waisel, 1974. Salt glands on leaves of Rhodes grass (Chlorisgayana Kth). Annals of Botany 38: 459–462.
Mackereth, F. J., H. J. Heron & J. F. Talling, 1978. Water Analysis: Some Revised Methods for Limnologists. Freshwater Biological Association, London.
Martin, G. D. & J. A. Coetzee, 2014. Competition between two aquatic macrophytes, Lagarosiphon major (Ridley) Moss (Hydrocharitaceae) and Myriophyllum spicatum Linnaeus (Haloragaceae) as influenced by substrate sediment and nutrients. Aquatic Botany 114: 1–11.
Medeiros, D. L., D. S. White & B. L. Howes, 2013. Replacement of Phragmites australis by Spartina alterniflora: the role of competition and salinity. Wetlands 33: 421–430.
Meerow, A. W., D. J. Lehmiller & J. L. Clayton, 2003. Phylogeny and biogeography of Crinum L. (Amaryllidaceae) inferred from nuclear and limited plastic non-coding DNA sequences. Botanical Journal of Linnean Society 141: 349–363.
Pennings, S. C. & M. D. Bertness, 1999. Using latitudinal variation to examine effects on climate on coastal salt marsh pattern and process. Current Topics in Wetland Biogeochemistry 3: 100–111.
Pennings, S. C., M. B. Grant & M. D. Bertness, 2005. Plant zonation in low-latitude salt marshes: disentangling the roles of flooding, salinity and competition. Journal of Ecology 93: 159–167.
Ribeiro, J. P., R. S. Matsumoto, L. K. Takao, A. C. Peret & M. I. S. Lima, 2011. Spatial distribution of Crinum americanum in the tropical blind estuary: hydrologic, edaphic and biotic drivers. Environmental and Experimental Botany 71: 287–291.
Ribeiro, J. P., R. S. Matsumoto, L. K. Takao & M. I. S. Lima, 2015. Plant zonation in a tropical irregular estuary: can large occurrence zones be explained by a tradeoff model? Brazilian Journal of Biology 75: 511–516.
Rodríguez-Gallego, L., V. Sabaj, S. Masciadri, C. Kruk, R. Arocena & D. Conde, 2015. Salinity as a major driver for submerged aquatic vegetation in coastal lagoons: a multi-year analysis in the subtropical Laguna de Rocha. Estuaries and Coasts 38: 451–465.
Smart, R. M. & J. W. Barko, 1980. Nitrogen nutrition and salinity tolerance of Distichlis spicata and Spartina alterniflora. Ecology 61: 630–638.
StatSoft, INC., 2005. Statistica (Data Analysis Software System), Version 7.1.
Stribling, J. M., 1997. The relative importance of sulfate availability in the growth of Spartina alterniflora and Spartina cynosuroides. Aquatic Botany 56: 131–143.
Suguio, K., 1973. Introdução à sedimentologia. EDUSP, São Paulo.
Tararam, A. S. & Y. Wakabara, 1987. Benthic fauna living on Spartina altemiflora of Cananéia estuarine lagoon (2S002′S–47°56′W). Boletim do Instituto Oceanográfico 35: 103–113.
Touchette, B. W., 2006. Salt tolerance in a Juncus roemerianus brackish marsh: spatial variations in plant water relations. Journal of Experimental Marine Biology and Ecology 337: 1–12.
van Gerven, L. P. A., J. J. M. de Klein, D. J. Gerla, B. W. Kooi, J. J. Kuiper & W. M. Mooij, 2015. Competition for light and nutrients in layered communities of aquatic plants. The American Naturalist 186: 72–83.
Zhi, Y., H. Li, S. An, L. Zhao, C. Zhou & Z. Deng, 2007. Inter-specific competition: Spartina alterniflora is replacing Spartina anglica in coastal China. Estuarine, Coastal and Shelf Science 74: 437–448.
Acknowledgements
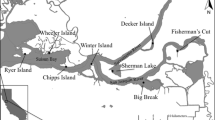
We thank Carlos Fernando Sanches and Amarílis Brandão de Paiva, M.S., for assistance with the experiment, Cristiane Akemi Umetsu, Ph.D., for helping in the statistical analyses and Leonardo Farage Cancian, Ph.D., for drawing up the map of the study area.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. T. O’Hare, F. C. Aguiar, E. S. Bakker & K. A. Wood / Plants in Aquatic Systems – a 21st Century Perspective
Rights and permissions
About this article
Cite this article
Nunes, L.S.C., Camargo, A.F.M. Do interspecific competition and salinity explain plant zonation in a tropical estuary?. Hydrobiologia 812, 67–77 (2018). https://doi.org/10.1007/s10750-016-2821-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2821-8