Abstract
We propose a simple and sensitive bioassay for analyzing zooplankton escape behavior and present results of its testing on four Daphnia species under varying temperature, and in either the presence or absence of chemical cues of fish predation. In the assay, the animals are blindly transferred with a pipette through subsequent containers and their final distributions are compared. For testing the effects of a single factor, comparing distributions of the animals is a sensitive tool to detect differences in escape ability. We propose single-value measures to be used in larger experiments with multigroup comparisons in order to enable studying global effects and reduce the loss of statistical significance due to correction for multiple testing. Our results show that if escape ability is considered an important fitness component, among the tested species, which were D. longispina, D. lumholtzi, D. magna, and D. pulicaria, the last may be the most vulnerable to increased fish predation associated with increased lake water temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ability to avoid predator attack is an important trait shaping individual fitness, population dynamics, and, in consequence, the structure of zooplankton communities. Perhaps, the most powerful amid planktonic prey defenses are pre-encounter mechanisms, with diel vertical migrations (DVM) being the most widespread in nature and also the most thoroughly studied (see for example reviews by Lampert, 1989; De Meester et al., 1999; Hays, 2003). However, if these defenses cannot be effective (e.g., when access to daytime deepwater refugium is denied by the hypolimnetic anoxia; Sakwinska & Dawidowicz, 2005), the post-encounter mechanisms shall prevail. Where the predator-to-prey size ratio is large enough to make futile morphological modifications that increase predator handling difficulty, like in the case of fish predation on zooplankton, escape may be the last line of defense. The importance of escape swimming to zooplankton predation avoidance has long been acknowledged, especially in copepods (e.g., Heath, 1993, Fields & Yen, 1997), and the contrast between copepods and cladocerans has initially diminished its role in Daphnia defense strategy (Drenner et al., 1978). This is not the case, however, and it was experimentally proven that Daphnia escape response can significantly reduce the predation success of visually feeding fish (Brewer et al., 1999). The escape performance varies between clones (genotypes), hence its variations can serve as an important natural selection factor in Daphnia (Brewer et al., 1999; Pijanowska et al., 2006).
Quantification of escape behavior in zooplankton has both gained considerable attention and posed technical difficulties to be surmounted with a sophisticated and costly equipment, such as high-speed video systems and the appropriate image-analyzing software (e.g., Buskey et al., 2002; Strickler & Balázsi, 2007; Bradley et al., 2013). Developing the ideas of Szlauer (1965), we propose a simple and sensitive bioassay for quantifying Daphnia ability to escape a suction-feeding predator and test it in four Daphnia species and under two sets of relevant environmental conditions: temperature and predator presence. Change in water temperature is expected to change mobility of the prey as it both affects its metabolic rate on the one hand, and hydrodynamic properties of water, i.e., density and viscosity, on the other. As the responses of metabolic rate to varying temperature are species specific, and also different sizes and morphologies affect hydrodynamics of Daphnia swimming, we hypothesized the effect of temperature on escape ability to be species dependent. As escape response has been shown to be dependent on animal alertness (e.g., Pijanowska et al., 2006), we also tested the effect of previous exposure to fish kairomone on the escape ability under the two tested temperatures.
Methods
Experiments
The test is performed by sequential transferring of animals with the use of pipette. The medium containing Daphnia initially fills the first container and is randomly transferred from the first to the second, from the second to the third, etc., until the tenth container, each time leaving 0.1 of the initial volume of the medium in the container (Fig. 1). Thus, by sequential transferring of the medium between containers, it becomes evenly distributed across ten containers. It is assumed that if the animals show no escape swimming, they will be evenly distributed with the medium at the end of experiment.
The test here was performed in 250-ml glasses, so at each transfer 25 ml was left in the source container. The volume moved decreased with subsequent transfers from 225 at first to 25 ml at the ninth transfer. A glass pipette of 5 mm in inner diameter and a rubber bulb were used. The estimated water velocity generated by the pipette strike was 30 cm s−1, which is within the range of velocities generated by suction-feeding planktivorous fish (Ferry-Graham et al., 2003). The volume of a single stroke of the pipette was about 2.5 ml, which is roughly equal to, e.g., suction volume of a 15-cm-long silver carp (Dong et al., 2006). To ensure random pipetting, at each transfer the source container was covered with an opaque wrapping, and the pipette tip was placed randomly at different spots in the container at subsequent strokes. The volume to be reached was marked on each target container (i.e., 225 ml on the second, 200 on the third, etc., and 25 on the tenth) to ensure that the right amount of medium is being transferred.
In the experiment, we used eight Daphnia clones, two of each of the four tested taxa: D. magna, D. pulicaria (sensu lato), D. longispina, and D. lumholtzi. D. magna clones originated from Binnensee (MABI) and a pond in Warsaw (MAKS). D. pulicaria originated from two distinct lineages: from lake Brome in Canada (PUBR) and a reservoir in Czech Republic (PUBH). D. longispina originated from lake Constance in Germany (LOCO) and lake Roś in Poland (LORO). D. lumholtzi originated from eutrophic lakes in Arizona (LUAR) and Texas (LUTE), USA. All clones were precultured in the laboratory for several generations.
A cohort of 100 young adult females of each clone was used in a single test. Kairomone-treated animals were incubated in 1 l of kairomone water for 24–48 h prior to the tests, and control animals were incubated in 1 l of control medium, i.e., aerated filtered lake water. Kairomone water was obtained by incubating for 24 h one crucian carp (Carassius carassius) in 5 l of aerated filtered lake water. Both media were supplemented with Scenedesmus obliquus as a food source provided at a concentration of 1 mg Corg l−1. Before the tests, animals were moved to the smaller amount (250 ml) of either fresh kairomone or control medium. Tests were performed in a well-lit chamber, in water baths of either 20 or 28 °C. High-temperature tests were performed after 2-h acclimation of the animals in 28 °C. All tests were run in triplicate.
Data analysis
We first tested the repeatability of the obtained results. Next, we simulated the transfers for a range of escapability index values and used the simulated distributions to estimate escapability of the Daphnia in the experiments. Finally, we performed pairwise comparisons of the empirical distributions and calculated single-value measures of escape ability, which enable multigroup comparisons and which we propose as simple alternatives for simulation-based estimates. All statistical analysis was performed in R (ver. 3.1.1).
Repeatability test
Kolmogorov–Smirnov tests were performed to compare the distributions between three replicates within treatments. Modified Bonferroni correction was applied to account for multiple testing (three tests within each treatment). The overall number of tests which returned a significant difference between replicate distributions for the whole dataset was recorded. The significance of this number was tested in a permutation test. 1000 permutations of the whole dataset, with distributions taken as data points, were performed. For each of the permuted datasets, the overall number of significant differences in the between-replicate comparisons was recorded. P value for the test was set as the frequency of obtaining an equal or lower number of significant results.
Transfer simulations and estimation of escapability
The probability of being transferred to the subsequent vessel increases in subsequent transfers, as the volume ratio of the transferred medium increases. Probability of being transferred by hazard is then P T(i) = V i+1 /V i , where V i+1 is the volume taken at i-th transfer and V i is the volume of the medium at the source container at the i-th transfer. Thus, in our setup P T = {9/10, 8/9, 7/8, 6/7, 5/6, 4/5, 3/4, 2/3, 1/2}. Animals that actively evade transfer reduce this probability by their specific factor. We thus introduce an escapability index which characterizes the animals in the tested cohort and obtains values E ∈ [0,1], where 0 means perfect avoidance of the pipette and 1 means being transferred with the medium randomly with no ability to escape. The number of animals caught in i-th transfer can then be modeled as N T(i) = N S (i) · P T (i) · E, where N S is the number of animals in the source container at each transfer.
We simulated the transfers for 101 values of escapability index: E = {0, 0.01, 0.02,…, 0.98, 0.99, 1}. To introduce variation to the model, we calculated the mean standard deviation of the observed probability of being caught, across the whole dataset, yet separately for each of the nine subsequent transfers (SDE(i)). Thus, for calculating N T(i) we used the empirically derived standard deviation, and instead of taking simply P T(i) · E, we randomly sampled the normal distribution of mean P T(i) · E and standard deviation SDE(i).
We pooled three simulated replicates, and the empirical distributions (of three replicates also pooled) were tested in Kolmogorov–Smirnov tests against data simulated for all 101 values of E. The E value for which Kolmogorov–Smirnov test returned the lowest value of D statistic was used as the estimated value of escapability index, Ê. After the simulation for each E value was repeated 1000 times, mean Ê was taken, and 2.5 and 97.5 percentiles designated the lower and upper bounds of 95% confidence intervals (CI) for the estimated values of escapability, respectively.
In addition, Ê was estimated as described above for the three replicates without pooling, and three-way ANOVA was performed on thus obtained mean Ê (three values per treatment). In addition, as three replicate values do not allow for much power of the analysis, we performed also ANOVA not on mean Ê, but on the direct estimates from 1000 simulations. ANOVA was performed on the results of 33 simulations and repeated 30 times (random 990 out of 1000 simulations).
Pairwise comparisons
We compared the distributions of animals at the end of the experiment between treatments in pairs within clones (three replicates pooled). This was done with the use of Kolmogorov–Smirnov test, followed by a sequential Bonferroni correction.
Distance traveled
This measure was based on how far each individual got transferred, i.e., to which container, one meaning that the individual escaped the first transfer and remained in the first container, ten meaning that the individual did not escape any transfer, got moved nine times, and reached the tenth container. Thus, it refers to catchability of the animals and lower values of the measure denote higher escape ability. Each of the 100 individuals in a test was assigned a value. Results from the three replicate tests were pooled. As the data conformed with Poisson distribution, GLM analysis was performed with Poisson error distribution and log link function, testing the effects of species, clone, kairomone, and temperature, followed by post hoc analysis.
Expected/observed
This measure was calculated as the ratio of the number of individuals expected to be left at each transfer under the assumption of no escape ability of the animals, i.e., proportional to the relative volume of water left, to the observed numbers of animals that escaped at each transfer. The expected number of animals was calculated as N e = N s · V l /V s, where N s is the number of individuals at the source container before the transfer, V l is the volume left in the source container after the transfer (25 ml each time in our case), and V s is the initial volume of water at the source container before the transfer. Again, lower values of the measure denote higher escape ability. The calculated ratios were analyzed with the use of three-way ANOVA with the subsequent transfer number (1–9) as a covariate, followed by post hoc analysis.
Results
Repeatability test
Across the whole dataset, there were seven between-replicate comparisons that returned a significant difference (out of 96). All 1000 permuted datasets returned higher values for this number. Variability between replicate distributions was lower than expected (P < 0.001), which signifies high repeatability of the results.
Estimated escapability
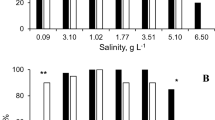
The estimated escapability (Ê) was significantly lower than 1 in all clones and treatments (Table 1). The values ranged from 0.698 in PUBH to 0.95 in LORO, both in control treatment. According to the conservative estimates of confidence interval bounds for Ê, in clones LUAR, MABI, and PUBH escapability significantly increased under higher temperature, and in MABI it increased in response to kairomone treatment at both temperatures (Fig. 2a). ANOVA performed on mean Ê estimated for three simulated replicates without pooling returned significant effect of temperature (F = 19.94, df = 1, P < 0.0001), treatment (F = 4.67, df = 1, P = 0.033), and species × treatment interaction (F = 3.92, df = 3, P = 0.011). The animals escaped more effectively in higher temperature and in kairomone treatment. ANOVA performed on the raw results of the simulations and repeated 30 times returned each time results equal to those obtained for the analysis of distance traveled measure (multiple group comparisons, see below).
A The estimated values of escapability (Ê) and their 95 % CI. C control, F kairomone treatment, at either 20 or 28 °C. B Distribution of Daphnia in subsequent glasses at the end of experiment. Results for three replicates are pooled. Each row presents results for a clone (LOCO to PUBR, see text for details). Significant differences are marked with asterisks: black between control and kairomone treatment at given temperature, white between 20 and 28 °C treatment at either control or kairomone treatment
Pairwise comparisons
All tested clones showed non-zero escape ability manifested by the right-skewed distributions of animals in glasses after the experiment (Kolmogorov–Smirnov tests against uniform distribution, D > 0.12, P < 0.0266). One clone was not reactive to the treatments applied (PUBR), while others reacted to either kairomone presence or temperature increase, or to both conditions. The between-treatment differences that proved significant after correction for multiple within-clone testing are shown in Fig. 2B.
Distance traveled
The between-treatment differences returned by the analysis of distance measure concorded the differences detected by comparing distributions and yet proved more sensitive as dealt better with corrections for multiple testing. In addition, post hoc analysis enabled between-group comparisons. According to this measure, D. pulicaria and D. lumholtzi showed higher overall escape ability (3.41 ± 0.05, mean ± SE, and 3.49 ± 0.05, respectively) than D. longispina and D. magna (3.61 ± 0.05 and 3.66 ± 0.05, respectively) (lower values of the distance measure denote higher escape ability). Temperature increase improved the overall Daphnia escape ability, yet it removed the advantage of D. pulicaria. At 20 °C, D. pulicaria showed higher escape ability than the other species, but this difference disappeared at 28°C, at which the escape ability of the species got homogenized. Kairomone presence improved escape ability in D. magna and D. longispina, thus reversing the relative differences in escape ability of the species. Escape ability ordered the species: D. pulicaria > D. lumholtzi > D. longispina > D. magna in the control treatment, but in kairomone presence D. magna escaped more efficiently than D. pulicaria. Within-species differences extracted from the global three-way interaction group comparison are presented in Fig. 3.
Catchability of four Daphnia species expressed as distance traveled in the experiment at 20 and 28 °C (mean ± SE). Pale tones control, dark tones kairomone. Significant differences are marked with asterisks: black between control and kairomone treatment at given temperature, white between 20 and 28 °C treatment at either control or kairomone treatment
Expected/observed
ANOVA performed on the probability of escaping returned highly significant effect of all tested factors, i.e., species (F = 8.28, df = 3, P < 0.0001), kairomone (F = 14.91, df = 1, P = 0.0001), and temperature (F = 30.22, df = 1, P < 0.0001), and also significant interaction of species with both kairomone (F = 3.60, df = 3, P = 0.0133) and temperature (F = 5,16, df = 3, P = 0.0015). According to this measure, D. longispina showed lower escape ability than the other species (0.73 ± 0.03, mean ± SE, vs. 0.65 ± 0.02 in D. magna, 0.64 ± 0.02 in D. lumholtzi, and 0.59 ± 0.02 in D. pulicaria) (lower values of the expected/observed measure denote higher escape ability). Temperature increase improved escape ability of all species except D. pulicaria, again, removing its advantage seen at 20 °C. As shown also by the distance measure, kairomone presence improved escape ability in D. magna and D. longispina, here removing differences between species seen in control treatment. Within-species differences extracted from the global three-way interaction group comparison are presented in Fig. 4.
Catchability of four Daphnia species expressed as a ratio of the expected to the observed number of individuals left after a transfer at 20 and 28 °C (mean ± SE). Pale tones control, dark tones kairomone. Significant differences are marked with asterisks: black between control and kairomone treatment at given temperature, white between 20 and 28 °C treatment at either control or kairomone treatment
Discussion
The bioassay for escape ability proposed in this study proved sensitive and its results repeatable. We were able to demonstrate between-species variations in evasion performance response to kairomone presence and the water temperature change. Besides, the bioassay is simple and cost effective and does not need any sophisticated equipment nor the qualified personnel. It is also not sensitive to a potential bias caused by various experimenters’ skills in catching live zooplankton with a pipette—each transfer was performed blindly and the animals were collected at random. This may ensure comparability of results of the bioassays performed by different persons. Although we cannot exclude that the animals were non-homogenously distributed in the sampling vessels, randomized sampling enabled to detect repeatable patterns in Daphnia distributions among the subsequent vessels at the end of experiment. This was confirmed by permutation test, as variability between replicate distributions was lower than expected in a randomized dataset. Also, the variability of single-value measures was significantly lower within treatments than between treatments, as indicated by ANOVA.
Simulation of the transfers followed by estimation of the escapability index (Ê), which was based on comparisons of simulated and empirical distributions, provided a reliable measure of ability to escape. Based on conservative estimates of confidence interval bounds, significant differences in escapability were found. Also ANOVA performed on either direct estimates or their means provided results conforming those obtained by pairwise comparisons of distributions and the proposed single-value measures, namely distance traveled and expected/observed number ratio. Yet, we suggest using these simple, solely empirical measures, which provided satisfying resolution and which are both more rooted in the data and do not demand advanced statistical procedures.
For testing the effects of a single factor in the proposed bioassay, comparing distributions of the animals was a sensitive tool to detect differences in escape ability. In larger experiments with multigroup comparisons, the use of a single-value measure, which enables studying global effects and reduces the loss of statistical significance due to correction for multiple testing, seems more convenient. Both proposed measures, the distance traveled and the ratio of the expected to the observed number of individuals left after a transfer, returned consistent results. However, the distance measure proved more sensitive. With this measure, we were able to detect statistical significance in responses in more cases, e.g., in D. magna reacting to both temperature and kairomone.
Ability to escape predator significantly increases the chances of Daphnia to survive under fish predation pressure and is thus an important component of their fitness (Brewer et al., 1999). The expected increase in water temperature of temperate lakes may increase planktivore pressure (Winder & Schindler, 2004), thus leading to increase in mortality rates in planktonic cladoceran populations and, ultimately, eliminating the most vulnerable species, large bodied Daphnia among them. We can also expect, though, that water warming will increase Daphnia mobility and their ability to actively evade predator attack. Indeed, our experiments showed differential increase in escape ability in the tested clones and species. This suggests that temperature increase may lead to differential changes in clone and species fitness and thus, indirectly, i.e., through changes in vulnerability to predation, may lead to microevolutionary changes in Daphnia populations within species, and also changes in species composition of zooplankton communities.
In addition, the experiment showed the effect of kairomone presence on escape ability, interacting with temperature and differing between species and clones, thus being genetically determined. This suggests that an increase in temperature might affect the efficiency to escape predator attack not only through changes in animal mobility (via increased metabolic rate coupled with decreased water density and viscosity), but also through genotype-specific changes in reactivity to chemical cues of predator presence and associated alertness (Brewer et al., 1999; Pijanowska et al., 2006).
Daphnia pulicaria was the most evasive species at low temperature. However, its increase from 20 to 28 °C did not increase its overall escape ability. This is not very surprising, as D. pulicaria is known to be a cold-adapted species (Palaima & Spitze, 2004). If evasiveness of the species does not follow the temperature-related increase in fish predation, this will directly lead to its relative decrease in fitness. This might particularly be the case of D. pulicaria fitness relative to the tropical invasive D. lumholtzi. The latter did escape more efficiently under temperature increase. Moreover, although neither D. pulicaria nor D. lumholtzi was behaviorally responsive to kairomone presence, D. lumholtzi has an alternative antipredator defense: fish kairomone-induced morphological change, i.e., long head and tail spine formation (Tollrian, 1994, Dzialowski et al., 2003). The other two species, D. longispina and D. magna, responded to both temperature increase and kairomone presence, which changed relative evasiveness of D. magna. It was the least evasive species in the absence of kairomone, yet in kairomone presence it escaped the simulated predator attack more efficiently than D. pulicaria. Interestingly, the observed behavioral responses were obviously not size dependent.
Conclusions
The proposed simple bioassay for analyzing zooplankton escape behavior, where the animals are blindly transferred with a pipette through subsequent containers, proved to be sensitive and efficient. Applying it, we showed how the rise in lake water temperature may lead to changes in relative fitness of clones and species through relative changes in escape ability, thus potentially leading to shifts in Daphnia assemblages’ composition. Moreover, the detected differential interactive responses to kairomone may add to this effect.
References
Bradley, C. J., J. R. Strickler, E. J. Buskey & P. H. Lenz, 2013. Swimming and escape behavior in two species of calanoid copepods from nauplius to adult. Journal of Plankton Research 35: 49–65.
Brewer, M. C., P. Dawidowicz & S. I. Dodson, 1999. Interactive effects of fish kairomone and light on Daphnia escape behavior. Journal of Plankton Research 21: 1317–1335.
Buskey, E. J., P. H. Lenz & D. K. Hartline, 2002. Escape behavior of planktonic copepods in response to hydrodynamic disturbances: high speed video analysis. Marine Ecology Progress Series 235: 135–146.
De Meester, L., P. Dawidowicz, E. Van Gool & C. J. Loose, 1999. Ecology and evolution of predator-induced behavior of zooplankton: depth selection behavior and diel vertical migration. In Tollrian, R. & C. D. Harvell (eds), The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton (NJ): 160–176.
Dong, S., D. Li, X. Bing, Q. Shi & F. Wang, 2006. Suction volume and filtering efficiency of silver carp (Hypophthalmichthys molitrix Val.) and bighead carp (Aristichthys nobilis Rich.). Journal of Fish Biology 41: 833–840.
Drenner, R. W., J. R. Strickler & W. J. O’Brien, 1978. Capture probability: the role of zooplankter escape in the selective feeding of planktivorous Fish. Journal of the Fisheries Research Board of Canada 35: 1370–1373.
Dzialowski, A. R., J. T. Lennon, W. J. O’Brien & V. H. Smith, 2003. Predator-induced phenotypic plasticity in the exotic cladoceran Daphnia lumholtzi. Freshwater Biology 48: 1593–1602.
Ferry-Graham, L. A., P. C. Wainwright & G. V. Lauder, 2003. Quantification of flow during suction feeding in bluegill sunfish. Zoology 106: 159–168.
Fields, D. M. & J. Yen, 1997. The escape behavior of marine copepods in response to a quantifiable fluid mechanical disturbance. Journal of Plankton Research 19: 1289–1304.
Hays, G. C., 2003. A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 503: 163–170.
Heath, M. R., 1993. The role of escape reactions in determining the size distribution of prey captured by herring larvae. Environmental Biology of Fishes 38: 331–344.
Lampert, W., 1989. The adaptive significance of diel vertical migration of zooplankton. Functional Ecology 3: 21–27.
Palaima, A. & K. Spitze, 2004. Is a jack-of-all-temperatures a master of none? An experimental test with Daphnia pulicaria (Crustacea: Cladocera). Evolutionary Ecology Research 6: 215–225.
Pijanowska, J., P. Dawidowicz & L. J. Weider, 2006. Predator-induced escape response in Daphnia. Archiv fur Hydrobiologie 167: 77–87.
Sakwińska, O. & P. Dawidowicz, 2005. Life history strategy and depth selection behavior as alternative antipredator defenses among natural Daphnia hyalina populations. Limnology and Oceanography 50: 1284–1289.
Strickler, J. R. & G. Balázsi, 2007. Planktonic copepods reacting selectively to hydrodynamic disturbances. Philosophical Transactions of The Royal Society B 362: 1947–1958.
Szlauer, L., 1965. The refuge ability of plankton animals before plankton eating animals. Polskie Archiwum Hydrobiologii 13: 89–95.
Tollrian, R., 1994. Fish-kairomone induced morphological changes in Daphnia lumholtzi (Sars). Archiv fur Hydrobiologie 130: 69–75.
Winder, M. & D. E. Schindler, 2004. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology 85: 2100–2106.
Acknowledgments
We are grateful to Jan Słotwiński for help in laboratory experiments and to Ralph Tollrian for providing us with D. lumholtzi clones. We thank Adam Petrusek and two anonymous reviewers for providing valuable comments that helped improve the manuscript. The study was financed by Polish National Science Centre Grant No. NN305 134440 to Piotr Dawidowicz.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Adam Petrusek & Piet Spaak / Proceedings of the 10th International Symposium on Cladocera
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pietrzak, B., Pijanowska, J. & Dawidowicz, P. The effect of temperature and kairomone on Daphnia escape ability: a simple bioassay. Hydrobiologia 798, 15–23 (2017). https://doi.org/10.1007/s10750-015-2539-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2539-z