Abstract
Haplochromine cichlids used to be the main prey of the introduced Nile perch, Lates niloticus, in Lake Victoria. After depletion of the haplochromine stocks at the end of the 1980s, Nile perch shifted to the shrimp Caridina nilotica and to a lesser degree to its own young and the cyprinid Rastrineobola argentea. In the present study, we investigated the Nile perch diet in the northern Mwanza Gulf after resurgence of some of the haplochromine species and compared it with data collected in the same area in 1988/1989. It became clear that haplochromines are again the major prey of Nile perch. The dietary shift from invertebrate feeding (shrimps) to feeding on fish (haplochromine cichlids) occurs at a smaller size than it did when Nile perch were taking primarily dagaa and juvenile Nile perch as their fish prey. The apparent preference for haplochromines as prey has reduced the degree of cannibalism considerably, which may have a positive impact on Nile perch recruitment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nile perch, Lates niloticus (L.), was introduced into Lake Victoria in the 1950s and early 1960s from lakes Turkana (Kenya) and Albert (Uganda) where it is native (Pringle, 2005). A dramatic increase of the Nile perch in the 1980s (Ogutu-Ohwayo, 1990a; Goudswaard et al., 2008) coincided with the disappearance of about 40% of the 500+ endemic haplochromine cichlid species (Witte et al., 1992). Concomitant with the disappearance of haplochromine species, increases of the cyprinid Rastrineobola argentea (Pellegrin), locally called dagaa, and of the shrimp Caridina nilotica (Roux) were noted (Wanink, 1999; Goudswaard et al., 2006). About a decade later Nile perch yields declined due to intense fishing, and resurgence of some haplochromine species was observed in the lake (Seehausen et al., 1997; Balirwa et al., 2003; Getabu et al., 2003; Witte et al., 2007).
During the period of the 1970s, when haplochromine cichlids were the dominant fish in the lake (Kudhongania & Cordone, 1974), they were the major prey of the Nile perch (Hamblyn, 1966; Gee, 1969). In the course of the 1980s, when the density of haplochromines in the sub-littoral and offshore waters declined to almost zero (Ogutu-Ohwayo, 1990a; Witte et al., 1992), Nile perch switched to shrimps, dagaa and to its own young (Mkumbo & Ligtvoet, 1992; Ogari & Dadzie, 1988; Ogutu-Ohwayo, 1990b; Katunzi et al., 2006). A similar shift in diet was noted in Lake Kyoga, where Nile perch had been introduced as well (Ogutu-Ohwayo, 1990b). In the Mwanza Gulf (Fig. 1), the switch from haplochromines to shrimps, juvenile Nile perch and dagaa, was rather abrupt, and occurred between September 1986 and June 1987 (Mkumbo & Ligtvoet, 1992). In the absence of haplochromine cichlids, shrimps seemed to be the preferred prey of Nile perch up to 30 cm TL in the sub-littoral areas of the Mwanza Gulf in 1988/1989. Even when juvenile Nile perch and dagaa were abundant, shrimps were eaten almost exclusively by this size class (Katunzi et al., 2006). However, it has been suggested that, with their recovery, haplochromines would take over the role as key prey again (Katunzi et al., 2006).
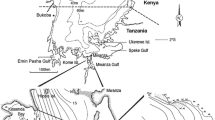
During the past 15 years, the abundance of some haplochromine species in the sub-littoral waters of the Mwanza Gulf has increased again and currently the total number of individuals of haplochromines is more than 50% of that in the 1970s (Witte et al., 2007; M. A. Kishe-Machumu, unpubl. data). Studies by Budeba and Cowx (2007) and by Ngupula and Mlaponi (2010) showed that after their resurgence in the Tanzanian waters of the lake, haplochromines became important prey of Nile perch again. However, these studies were mainly aimed at the role of the shrimp Caridina nilotica in the Nile perch diet and did not make comparisons with Nile perch diets in the past from the same lake areas. To examine explicitly whether Nile perch from sub-littoral waters in the Mwanza Gulf switch back to haplochromines as their major food source after the resurgence of some species, we first quantified the diet of Nile perch on a research transect in the Mwanza Gulf between Butimba Bay and Kissenda Bay (Fig. 1). We then compared these data to a subset of data that were collected in 1988–1998 by Katunzi et al. (2006) from an area that partially overlapped with our transect. To enable comparisons with earlier studies, we used both the frequency of occurrence method and the volumetric percentage method to characterize the Nile perch diet.
Materials and methods
Study area
The Nile perch samples were collected in the sub-littoral (6–20 m) waters on a research transect across the Mwanza Gulf, between Butimba Bay and Kissenda Bay (Fig. 1). The research transect is approximately 5 km long and six sampling stations (E, F, G, H, I and J) with depths ranging from 5.5–13 m were sampled. The bottom of these stations is covered with a thick deposit of organic mud (Witte, 1981).
Sampling techniques
A small trawler, used for sampling haplochromine cichlids, was powered by a 25-hp outboard engine and towed a bottom trawl net with a 4.6-m head-rope and a 5-mm cod-end mesh. It only caught small-sized Nile perch (up to ca. 30 cm TL). Therefore, large trawlers (165–215 hp) using nets with a head-rope of 22–27 m and a 50–100-mm stretched cod-end mesh were used to catch larger Nile perch. Trawl-shots lasted for 10 and 30 min with the small and large trawlers, respectively. Sampling in 2006 and 2008 was mostly conducted in the morning hours between 08.00 and 12.00. The only exception was 7 March 2008, when 25 Nile perch with food in their stomachs were collected with a large trawler between 14.50 and 18.00.
With the small trawler, a trawl-shot was made at each station once per month in 2006 and once per two months in 2008. The large trawlers were used in January, March, May and December 2006 and in March, August, October and December 2008. In each month three to four hauls were made on one day, and each trawl-shot covered two to three stations on the transect. For data analysis, the Nile perch samples from the small and large trawlers were combined.
Biotic data and stomach examination
Total length (TL) of Nile perch was measured to the nearest centimetre (digits ending at ≥0.5 were rounded to the nearest cm above). Depending on the size of the catch, stomach contents of all Nile perch ≤20 cm TL, or of a sub-sample, were analysed, while all fish >20 cm TL were all analysed. When using a large trawler, identification of stomach contents was performed on board, immediately after each haul. In case of the small trawler, fish were kept on ice and analysis was performed in the laboratory on the same day. The entire stomach of each fish was removed. Before opening the stomach, its fullness was estimated at five levels; 0, 25, 50, 75 and 100%. The prey items were identified to the lowest possible taxonomic level. Small prey items like zooplankton and midge larvae that could only be observed with the aid of a microscope were discarded in this study, as we were mainly interested in the contribution of haplochromines and other large prey items to the diet. Moreover, Katunzi et al. (2006) showed that they were relatively unimportant prey types for Nile perch at the research transect (their station 3). Zooplankton was only eaten by fish <5 cm TL (frequency of occurrence <15%), and midge larvae mainly by fish <10 cm (frequency of occurrence <30%).
The frequency of occurrence of each food item was expressed as the percentage of fish containing that food item out of the total number of fish containing food. Volume per food type was estimated for each fish separately as a percentage of the total volume of food it had ingested and then a correction on volume per food type was made for the percentage of fullness of the stomach. This implied that the sum of the volume percentages of all food types, together with the empty part added up to 100%.
For calculating frequencies of occurrence and volume percentages of prey items, fish with empty stomachs were discarded and those filled less than 25% were discarded for calculating volume percentages. In total, 1,073 fish (2.5–114 cm TL) from 2006 and 2008 with food in their stomachs were used for calculating frequencies of occurrence of prey items, whereas 1,070 were used for volume percentages. Fish were grouped into size classes of 5 cm. The minimum number of individuals per length class was 11 (46–50 cm TL) and maximum number was 242 (11–15 cm TL).
Comparison with data collected in 1988/1989
For a comparison of Nile perch diets in 2006/2008 with those in 1988/1989, we used fish up to 30 cm TL, as this was the size range studied by Katunzi et al. (2006). From the latter study, we only used Nile perch containing food (n = 1,392) that were collected at station 3 (12–16 m deep), whereas from the present study we used Nile perch containing food (n = 639) from the deeper stations of the transect (F, G, H and I; 9.5–13 m), as these stations roughly overlapped with station 3 in Katunzi et al. (2006). Data collected by Katunzi et al. (2006) for 1 cm classes of Nile perch were lumped as 5 cm classes to make them comparable with those in the present study.
Statistics
A Pearson correlation coefficient was calculated between the percentages of empty stomachs and fish size classes (5 cm) for all stations combined. The Jonckheere–Terpstra test was used to test for trends of prey items amongst size classes. A Chi-Square test was used for testing if differences in frequencies of occurrence of food items between 1988/1989 and 2006/2008 were significant. Sequential Bonferroni corrections were applied in case of repeated tests. Statistical tests were performed with SPSS 17.0 for Windows.
Results
Frequency of occurrence of prey items
On average, 28.4 ± 11.5% of all studied stomachs were empty. The average percentage of empty stomachs was positively correlated with Nile perch length class (Pearson correlation coefficient: r = 0.868; P = 0.001; n = 11). The average percentage of empty stomachs in Nile perch from 1 to 30 cm was 21.1 ± 8.7%.
The shrimp C. nilotica and haplochromine cichlids were the most common prey types in Nile perch (Table 1). With increasing size of Nile perch, the frequency of occurrence of shrimps decreased significantly (98–0%; Jonckheere–Terpstra test: P ≤ 0.001; n = 1,073), whereas that of haplochromines increased significantly (0–60%; Jonckheere–Terpstra test: P ≤ 0.001; n = 1,073). Dagaa was eaten by a relatively small proportion of all size classes of Nile perch. Juveniles of its own species were found in low proportions of Nile perch ≥11 cm TL. For dagaa and juvenile Nile perch, the frequency of occurrence increased significantly with Nile perch size (Jonckheere–Terpstra test: P ≤ 0.001; n = 1,073 for both prey types), especially for the size classes ≥26 cm TL, reaching maxima of about 20% in fish >50 cm (Table 1). Other fish taxa were rather rare in the Nile perch diet and mainly comprised juvenile Oreochromis niloticus (L.) and Barbus spp. A relatively large percentage of Nile perch contained fish remains that could not be further identified as they were too far digested (Table 1). The frequencies of unidentifiable fish also increased significantly with Nile perch size (Jonckheere–Terpstra test: P = 0.011; n = 1,073). The highest frequencies (10–11%) of dragonfly nymphs (Odonata) and molluscs were occurring in the intermediate size classes (Table 1). Two large fish (one of 46–50 cm and one ≥51 cm) contained crab and plant remains in their stomachs, listed as ‘other prey’.
Volume percentages of food items
With the volume percentage method, similar results as with the frequency of occurrence were obtained (Table 2). Shrimps dominated the diet (40–86%) in Nile perch up to 20 cm TL, whereas haplochromines dominated (33–46%) in Nile perch from 21 cm onwards. The contribution of juvenile Nile perch to the diet increased slowly with predator length, but was never more than 9%; the unidentifiable fish species reached a maximum of 12% in size class 46–50 cm TL. Insects, molluscs, dagaa and other fish taxa never contributed more than 9% to the diet of each size class. The volume percentages of ‘other prey’ were negligible.
Comparison of data collected in 1988/1989 and 2006/2008
The proportion of Odonata in the diet of Nile perch was independent of time period for all size classes, except for Nile perch of 11–15 cm, where the proportion was higher in 2006/2008 (Fig. 2; Chi-Square: P = 0.009). For the other size classes, the difference in the frequency of occurrence of Odonata was not significant (Chi-Square: P ranging from 0.102 to 0.777). However, all other food items showed striking differences amongst both periods. In 2006/2008, Nile perch up to 20 cm TL ate shrimps more frequently than in 1988/1989 (Chi-Square: P ≤ 0.009), whereas the reverse was true for Nile perch of 21–30 cm TL (Chi-Square: P ≤ 0.004; Fig. 2). After Bonferroni corrections, the frequencies of occurrence of dagaa in Nile perch from 16 to 30 cm TL were strikingly lower in 2006/2008 than in 1988/1989 (Chi-Square: P = 0.032–0.191 for size range 1–15 cm and P ≤ 0.006 for size range 16–30 cm; Fig. 2). Moreover, for Nile perch ranging from 1 to 30 cm TL, in 1988/1989 the frequency of occurrence of dagaa increased significantly with Nile perch size (Jonckheere–Terpstra test: P ≤ 0.001; n = 1,392), but this was not the case in 2006/2008 (Jonckheere–Terpstra test: P = 0.373; n = 639). In 1988/1989, juvenile Nile perch were present in 25–30% of the stomachs of their conspecifics larger than 6 cm, whereas in 2006/2008 this had declined to less than 3% (Fig. 2; Chi-Square: P ≤ 0.001 for all Nile perch classes between 6 and 30 cm). In 1988/1989, haplochromines were virtually absent in sub-littoral waters in the northern part of the Mwanza Gulf and consequently also absent in Nile perch stomachs. Molluscs were not encountered in the diet of Nile perch from 1988/1989. The average percentage of empty stomachs in Nile perch from 1 to 30 cm in 2006/2008 (21.1 ± 8.7%) was similar to that found during the non-haplochromine diet regime (viz. 21.2 ± 5.6%) in 1988/1989 by Katunzi et al. (2006).
Frequency of occurrence of the main prey types of Nile perch up to 30 cm TL (per 5-cm size class) in 1988/1989 (n = 1,392) and 2006/2008 (n = 639): a shrimps, b dagaa, c Odonata nymphs, d haplochromines, e Nile perch. For 2006/2008, only Nile perch was used collected at stations F, G, H and I, which approximately cover station 3 sampled by Katunzi et al. (2006) in 1988/1989
Discussion
Obviously, shrimps and haplochromines were the key prey of Nile perch in the northern part of the Mwanza Gulf in 2006/2008. In Nile perch up to 20 cm, the dominant prey items were shrimps and in the larger size classes they were haplochromines. Though unidentified fishes also contributed significantly to the frequency of occurrence of prey types, their contribution to the volume percentages was much smaller (cf. Tables 1, 2). Moreover, it should be noted that most likely a major part of the unidentified fishes were haplochromines that were too far digested to be identified. During a survey in the entire Tanzanian sector of the lake in November 2006, it was found that shrimps dominated in the diet of Nile perch <50 cm TL by contributing 54% by volume, whereas haplochromines made up 41% of the diet of Nile perch >50 cm TL (Ngupula & Mlaponi, 2010). Our data indicate that in the northern part of the Mwanza Gulf the switch from shrimps to haplochromines as dominant prey occurred already at the size of about 20 cm TL. Similar results were found in 1999–2002 by Budeba & Cowx (2007) for their zone A, which included the Speke Gulf, the Mwanza Gulf and the offshore area between Kome Island and Ukerewe Island. In the other two zones (zone B near Musoma and zone C covering the south-western part of the Tanzanian waters) haplochromines only became the dominant prey in Nile perch >50–60 cm TL. As there is no information on haplochromine and shrimp densities in these areas, the cause of this delayed switch is not clear.
In September 1986, when haplochromine catches in the Mwanza Gulf had decreased to ca. 60 kg h−1 (from ca. 1,500 kg h−1 in the 1970s), haplochromines still were the most frequently occurring prey (>60%) in Nile perch of 15–40 cm TL (Mkumbo & Ligtvoet, 1992). Between June 1987 and August 1988, when haplochromines had virtually vanished from the trawl catches (Goudswaard et al., 2008), the frequency of occurrence of haplochromines in Nile perch of 15–40 cm TL had decreased to less than 5% (Mkumbo & Ligtvoet, 1992). Finally, in the period of September 1988 to September 1989, no haplochromines at all were found in Nile perch up to 30 cm TL (Fig. 2; Katunzi et al., 2006). The shift from haplochromines to shrimps as the main prey between 1986 and 1989 in the Mwanza Gulf was clearly due to the decline of the haplochromines (Witte et al., 1992; Goudswaard et al., 2008) and the concomitant increase of shrimps (Goudswaard et al., 2006).
Katunzi et al. (2006) found that in 1988/1989 at station 3 in the Mwanza Gulf, Nile perch up to 30 cm TL, in addition to shrimps (the most common prey), fed on juvenile Nile perch (≤5 cm TL), and with lower frequency on dagaa. However, it appeared that in seasons with high abundance of shrimps, juvenile Nile perch (and dagaa) were neglected, even if their abundance was high. Though the biomass of dagaa increased in the lake during the past decade (Tumwebaze et al., 2007; Witte et al., 2009), their contribution to the Nile perch diet in 2006/2008 was much lower than in 1988/1989 and never exceeded 3% by volume percentage (Table 2). Our current observations suggest that Nile perch >20 cm TL prefer haplochromines over shrimps. From 40 cm TL onwards, no shrimps at all were present in the diet, whereas during the second half of the 1980s, when haplochromines were absent, shrimps were eaten by Nile perch up to 60 cm TL (Ogutu-Ohwayo, 1990b; Mkumbo & Ligtvoet, 1992) and in the Nyanza Gulf (Kenya) even by fish of more than 1 m (Hughes, 1986; Ogari & Dadzie, 1988). Concordantly, the frequency of occurrence of shrimps in the diet of Nile perch up to 30 cm TL did not show a trend across size classes in 1988/1989 when haplochromines were absent, but a significant negative trend was found after the recovery of the haplochromines in 2006/2008 (Fig. 2).
In Lake Kyoga, where the upsurge and decline of the Nile perch occurred about a decade earlier than in Lake Victoria (Ogutu-Ohwayo, 1990a, 2004), a similar pattern was observed as in the Mwanza Gulf; shrimps were more important than haplochromines in the diet of Nile perch <20 cm and of 20–59 cm TL in 1978–1980. However, in 1991–1993 haplochromines dominated in the diet of Nile perch of these two size classes, as well as in the size class of 60–100 cm TL (Ogutu-Ohwayo, 2004).
In Lake Nabugabo, where shrimps appeared to be absent, dagaa and juvenile Nile perch were the main prey types before the mid-1990s, when haplochromines were extremely rare in the lake (Ogutu-Ohwayo, 1993; Schofield & Chapman, 1999). After the mid-1990s, when haplochromines were resurging in Lake Nabugabo, the diet of the Nile perch changed and in 2000 and 2007 it was dominated by haplochromine cichlids (Paterson & Chapman, 2009). Notably, the shift from invertebrates to fish occurred at a smaller size (between 10 and 15 cm TL) in case of haplochromines than in case of dagaa and juvenile Nile perch (between 30 and 35 cm TL; Paterson & Chapman, 2009). This agrees with our observations in the Mwanza Gulf, where in 1988/1989 shrimps were eaten more frequently than fish by Nile perch up to at least 30 cm TL, while in 2006/2008 haplochromines became the most frequently eaten prey from 21 cm onwards. In conclusion, it seems that haplochromines are the preferred prey of Nile perch that are large enough to feed on them.
Between 1987 and 1989, when haplochromines had virtually vanished from the Mwanza Gulf (Witte et al., 1992; 2007), cannibalism became important in Nile perch ≥5 cm TL (Mkumbo & Ligtvoet, 1992; Katunzi et al., 2006), whereas it strongly decreased after resurgence of the haplochromines. Also in other parts of Lake Victoria and in Lake Kyoga, Nile perch began feeding on their young only when haplochromine species were depleted (Ogutu-Ohwayo, 1993). Hopson (1972) stated that cannibalism in Lake Chad may have resulted partly from the scarcity of preferable prey items. During the past years, there has been some worry that the resurging haplochromines may do harm to the Nile perch stocks by feeding on Nile perch eggs and larvae (e.g. Kolding et al., 2008). Though this may be the case (Goudswaard et al., 2008), the data of our study indicate that the preference of Nile perch for haplochromines instead of their own young, reduces cannibalism. This may counterbalance the loss of eggs and larvae or even enhance rates of recruitment of Nile perch, which is of great importance for a sound fishery.
Conclusion
Based on a modelling study by Kaufman & Schwarz (2002), indicating that Nile perch prefer and grow fastest on a haplochromine prey base, it has been suggested that conservation of biodiversity and fishery sustainability may not have to be antitheses in the management of Lake Victoria (Balirwa et al., 2003). The observed reduction of cannibalism by Nile perch when haplochromines are available seems to support this suggestion.
Since haplochromines, are now taken as a by-catch in the dagaa fishery, management measures in dagaa fishery should take haplochromines into account. If the recovery of the haplochromine cichlids will continue, high dependence of the Nile perch on haplochromines and low rates of cannibalism, may contribute to another round of the Nile perch success in Lake Victoria.
References
Balirwa, J. S., C. A. Chapman, L. J. Chapman, I. G. Cowx, K. Geheb, L. Kaufman, R. H. Lowe-McConnell, O. Seehausen, J. H. Wanink, R. L. Welcomme & F. Witte, 2003. Biodiversity and fishery sustainability in the Lake Victoria basin: an unexpected marriage? Bioscience 53: 703–715.
Budeba, Y. L. & I. G. Cowx, 2007. The role of the freshwater shrimp Caridina nilotica (Roux) in the diet of the major commercial fish species in Lake Victoria, Tanzania. Aquatic Ecosystem Health and Management 10: 368–380.
Gee, J. M., 1969. A comparison of certain aspects of the biology of Lates niloticus (Linnaeus) in some East African lakes. Revue de Zoologie et Botanique Africaines 80: 244–262.
Getabu, A., R. Tumwebaze & D. N. MacLennan, 2003. Spatial distribution and temporal change in the fish populations of Lake Victoria. Aquatic Living Resources 16: 159–165.
Goudswaard, P. C., F. Witte & J. H. Wanink, 2006. The shrimp Caridina nilotica in Lake Victoria (East Africa), before and after the Nile perch increase. Hydrobiologia 563: 31–44.
Goudswaard, P. C., F. Witte & E. F. B. Katunzi, 2008. The invasion of an introduced predator, Nile perch (Lates niloticus, L.) in Lake Victoria (East Africa): chronology and causes. Environmental Biology of Fishes 81: 127–139.
Hamblyn, E. L., 1966. The food and feeding habits of Nile perch: Lates niloticus (Linné) (Pisces: Centropomidae). Revue de Zoologie et Botanique Africaines 74: 1–28.
Hopson, A. J., 1972. A study of the Nile perch (Lates niloticus L., Pisces: Centropomidae) in Lake Chad. Overseas Research Publications, London.
Hughes, N. F., 1986. Changes in the feeding biology of the Nile perch, Lates niloticus, in Lake Victoria since its introduction in 1960, and its impact on the native fish community of the Nyanza Gulf. Journal of Fish Biology 29: 541–548.
Katunzi, E. F. B., W. L. T. van Densen, J. H. Wanink & F. Witte, 2006. Spatial and seasonal patterns in the feeding habits of juvenile Lates niloticus (L.), in the Mwanza Gulf of Lake Victoria. Hydrobiologia 568: 121–133.
Kaufman, L. & J. Schwarz, 2002. Nile perch population dynamics in Lake Victoria: implications for management and conservation. In Ruth, M. & J. Lindholm (eds), Dynamic Modelling for Marine Conservation. Springer, New York: 257–313.
Kolding, J., P. van Zwieten, O. C. Mkumbo, G. Silsbe & R. E. Hecky, 2008. Are the Lake Victoria fisheries threatened by exploitation or eutrophication? Towards an ecosystem based approach to management. In Bianchi, G. & H. R. Skjodal (eds), The Ecosystem Approach to Fisheries. CAB International, Wallingford: 309–354.
Kudhongania, A. W. & A. J. Cordone, 1974. Batho-spatial distribution patterns and biomass estimates of the major demersal fishes in Lake Victoria. The African Journal of Tropical Hydrobiology and Fisheries 3: 15–31.
Mkumbo, O. C. & W. Ligtvoet, 1992. Changes in the diet of Nile perch, Lates niloticus (L), in the Mwanza Gulf of Lake Victoria. Hydrobiologia 232: 79–84.
Ngupula, G. W. & E. Mlaponi, 2010. Changes in the abundance of Nile shrimp, Caridina nilotica (Roux) following the decline of Nile perch and recovery of native haplochromine fishes, Lake Victoria, Tanzanian waters. Aquatic Ecosystem Health and Management 13: 196–202.
Ogari, J. & S. Dadzie, 1988. The food of Nile perch, Lates niloticus (L.), after the disappearance of the haplochromine cichlids in the Nyanza Gulf of Lake Victoria (Kenya). Journal of Fish Biology 32: 571–577.
Ogutu-Ohwayo, R., 1990a. The decline of the native fishes of Lakes Victoria and Kyoga (East Africa) and the impact of introduced species, especially the Nile perch, Lates niloticus and the Nile tilapia, Oreochromis niloticus. Environmental Biology of Fishes 27: 81–96.
Ogutu-Ohwayo, R., 1990b. Changes in the prey ingested and the variations in the Nile perch and other fish stocks of Lake Kyoga and the northern waters of Lake Victoria (Uganda). Journal of Fish Biology 37: 55–63.
Ogutu-Ohwayo, R., 1993. The effects of predation by the Nile perch Lates niloticus L., on the fishes of Lake Nabugabo, with suggestions for conservation of endangered endemic cichlids. Conservation Biology 7: 701–711.
Ogutu-Ohwayo, R., 2004. Management of the Nile perch, Lates niloticus fishery in Lake Victoria in light of the changes in its life history characteristics. African Journal of Ecology 42: 306–314.
Paterson, J. A. & L. J. Chapman, 2009. Fishing down and fishing hard: ecological change in the Nile perch of Lake Nabugabo, Uganda. Ecology of Freshwater Fish 18: 380–394.
Pringle, R. M., 2005. The origins of the Nile perch in Lake Victoria. Bioscience 55: 780–787.
Schofield, P. J. & L. J. Chapman, 1999. Interactions between Nile perch, Lates niloticus, and other fishes in Lake Nabugabo. Environmental Biology of Fishes 55: 343–358.
Seehausen, O., F. Witte, E. F. B. Katunzi, J. Smits & N. Bouton, 1997. Patterns of the remnant cichlid fauna in southern Lake Victoria. Conservation Biology 11: 890–904.
Tumwebaze, R., I. Cowx, S. Ridgway, A. Getabu & D. N. MacLennan, 2007. Spatial and temporal changes in the distribution of Rastrineobola argentea in Lake Victoria. Aquatic Ecosystem Health & Management 10: 398–406.
Wanink, J. H., 1999. Prospects for the fishery on the small pelagic Rastrineobola argentea in Lake Victoria. Hydrobiologia 407: 183–189.
Witte, F., 1981. Initial results of the ecological survey of the haplochromine cichlid fishes from the Mwanza Gulf of Lake Victoria, Tanzania: breeding patterns, trophic and species distribution. Netherlands Journal of Zoology 31: 175–202.
Witte, F., T. Goldschmidt, J. Wanink, M. van Oijen, K. Goudswaard, E. Witte-Maas & N. Bouton, 1992. The destruction of an endemic species flock: quantitative data on the decline of the haplochromine cichlids of Lake Victoria. Environmental Biology of Fishes 34: 1–28.
Witte, F., J. H. Wanink & M. A. Kishe-Machumu, 2007. Species distinction and the biodiversity crisis in Lake Victoria. Transactions of the American Fisheries Society 136: 1146–1159.
Witte, F., M. de Graaf, O. Mkumbo, A. I. El-Moghraby & F. A. Sibbbing, 2009. Fisheries in the Nile system. In Dumont, H. J. (ed.), The Nile: Origin, Environments, Limnology and Human Use. Springer, Berlin: 723–747.
Acknowledgments
We would like to thank the following organisations that funded this study: the Netherlands Foundation for the Advancement of Tropical Research (WOTRO; grants W 87-189, W 84-488 and WB84-587), the Netherlands Organization for International Cooperation in Higher Education (NUFFIC), the International Foundation for Sciences (IFS), the Schure Beijerinck-Popping Fonds and the section DPO of the Netherlands Ministry of Foreign Affairs. We are also grateful to the crew members of the small trawler; Mhoja Kayeba, Mohamed Haruna and Rajabu Machage, the master fishermen; Raphael Mamelela and Gabriel Mbaya of the larger trawlers (R.V. Lake Victoria Explorer and M.V. Mdiria) and all other crew members for making sampling successful.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: C. Sturmbauer, C. Albrecht, S. Trajanovski & T. Wilke / Evolution and Biodiversity in Ancient Lakes
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kishe-Machumu, M.A., Witte, F., Wanink, J.H. et al. The diet of Nile perch, Lates niloticus (L.) after resurgence of haplochromine cichlids in the Mwanza Gulf of Lake Victoria. Hydrobiologia 682, 111–119 (2012). https://doi.org/10.1007/s10750-011-0822-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0822-1