Abstract
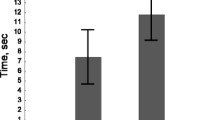
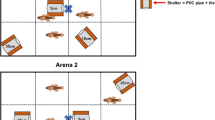
Parasites with a complex life cycle are supposed to influence the behaviour of their intermediate host in such a way that the transmission to the final host is enhanced, but reduced to non-hosts. Here, we examined whether the trophically transmitted bird parasite Polymorphus minutus increases the antipredator response of its intermediate host, the freshwater amphipod Gammarus pulex to fish cues, i.e. non-host cues (‘increased host abilities hypothesis’). Aggregation behaviour and reduced activity are assumed to decrease the predation risk of gammarids by fishes. Uninfected G. pulex are known to aggregate in the presence of a fish predator. In the present study, gammarids were allowed to choose either to join a group of conspecifics or to stay solitary (experiment 1) or between two groups differing in infection status (experiment 2), both in the presence or absence of fish odour. The perception of the groups was limited to mainly olfactory cues. Contrary to the ‘increased host abilities hypothesis’, in infected gammarids of experiment 1, fish cues induced similar aggregation behaviour as in their uninfected conspecifics. In experiment 2, uninfected as well as infected gammarids did not significantly discriminate between infected and uninfected groups. Although only uninfected gammarids reduced their activity in the presence of predator cues, infected G. pulex were generally less active than uninfected conspecifics. This might suggest that P. minutus manipulates rather the general anti-predator behaviour than the plastic response to predation risk.

Similar content being viewed by others
References
Andersson, K. G., C. Brönmark, J. Herrmann, B. Malmqvist, C. Otto & P. Sjörström, 1986. Presence of sculpins (Cottus gobio) reduces drift and activity of Gammarus pulex (Amphipoda). Hydrobiologia 133: 209–215.
Bakker, T. C. M., D. Mazzi & S. Zala, 1997. Parasite-induced changes in behavior and color make Gammarus pulex more prone to fish predation. Ecology 78: 1098–1104.
Baldauf, S. A., T. Thünken, J. G. Frommen, T. C. M. Bakker, O. Heupel & H. Kullmann, 2007. Infection with an acanthocephalan manipulates an amphipod’s reaction to a fish predator’s odours. International Journal for Parasitology 37: 61–65.
Barber, I., F. A. Huntingford & D. W. T. Crompton, 1995. The effect of hunger and cestode parasitism on the shoaling decisions of small fresh-water fish. Journal of Fish Biology 47: 524–536.
Bauer, A., E. R. Haine, M. J. Perrot-Minnot & T. Rigaud, 2005. The acanthocephalan parasite Polymorphus minutus alters the geotactic and clinging behaviours of two sympatric amphipod hosts: the native Gammarus pulex and the invasive Gammarus roeseli. Journal of Zoology 267: 39–43.
Baumgärtner, D., A. D. Jungbluth, U. Koch & E. von Elert, 2002. Effects of infochemicals on microhabitat choice by the freshwater amphipod Gammarus roeseli. Archiv für Hydrobiologie 155: 353–367.
Benesh, D. P., T. Hasu, O. Seppälä & E. T. Valtonen, 2009. Seasonal changes in host phenotype manipulation by an acanthocephalan: time to be transmitted? Parasitology 136: 219–230.
Bethel, W. M. & J. C. Holmes, 1973. Altered evasive behavior and responses to light in amphipods harboring acanthocephalan cystacanths. Journal of Parasitology 59: 945–956.
Brock, V. E. & R. H. Riffenburgh, 1960. Fish schooling: a possible factor in reducing predation. Journal du Conseil, Conseil International pour l’Exploration de la Mer 25: 307–317.
Brown, A. F. & D. Pascoe, 1989. Parasitism and host sensitivity to cadmium – an acanthocephalan infection of fresh-water amphipod Gammarus pulex. Journal of Applied Ecology 26: 473–487.
Cézilly, F., A. Gregoire & A. Bertin, 2000. Conflict between co-occurring manipulative parasites? An experimental study of the joint influence of two acanthocephalan parasites on the behaviour of Gammarus pulex. Parasitology 120: 625–630.
Crawley, M. J., 2005. Statistics: An Introduction Using R. Chichester (United Kingdom). Wiley, Chichester, UK.
Crompton, D. W. T. & B. B. Nickol, 1985. Biology of the Acanthocephala. Cambridge University Press, Cambridge.
Dahl, J., P. A. Nilsson & L. B. Pettersson, 1998. Against the flow: chemical detection of downstream predators in running waters. Proceedings of the Royal Society of London Series B 265: 1339–1344.
Dezfuli, B. S., B. J. Maynard & T. A. Wellnitz, 2003. Activity levels and predator detection by amphipods infected with an acanthocephalan parasite, Pomphorhynchus laevis. Folia Parasitologica 50: 129–134.
Engqvist, L., 2005. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Animal Behaviour 70: 967–971.
Frommen, J. G., M. Hiermes & T. C. M. Bakker, 2009. Disentangling the effects of group size and density on shoaling decisions of three-spined sticklebacks (Gasterosteus aculeatus). Behavioural Ecology and Sociobiology 63: 1141–1148.
Gerritsen, J. & J. R. Strickler, 1977. Encounter probabilities and community structure in zooplankton: a mathematical model. Journal of the Fisheries Research Board of Canada 34: 73–82.
Jakobsen, P. J. & C. Wedekind, 1998. Copepod reaction to odor stimuli influenced by cestode infection. Behavioral Ecology 9: 414–418.
Kaldonski, N., M. J. Perrot-Minnot & F. Cezilly, 2007. Differential influence of two acanthocephalan parasites on the antipredator behaviour of their common intermediate host. Animal Behaviour 74: 1311–1317.
Kaldonski, N., M. J. Perrot-Minnot, S. Motreuil & F. Cézilly, 2008. Infection with acanthocephalans increases the vulnerability of Gammarus pulex (Crustacea, Arnphipoda) to non-host invertebrate predators. Parasitology 135: 627–632.
Kennedy, C. R., P. F. Broughton & P. M. Hine, 1978. Status of brown and rainbow trout, Salmo trutta and S. gairdneri as hosts of acanthocephalan, Pomphorhynchus laevis. Journal of Fish Biology 13: 265–275.
Krakauer, D. C., 1995. Groups confuse predators by exploiting perceptual bottlenecks – a connectionist model of the confusion effect. Behavioral Ecology and Sociobiology 36: 421–429.
Krang, A. S. & S. P. Baden, 2004. The ability of the amphipod Corophium volutator (Pallas) to follow chemical signals from con-specifics. Journal of Experimental Marine Biology and Ecology 310: 195–206.
Krause, J. & J.-G. J. Godin, 1994. Shoal choice in the banded killifish (Fundulus diaphanus, Teleostei, Cyprinodontidae) – effects of predation risk, fish size, species composition and size of shoals. Ethology 98: 128–136.
Krause, J. & G. D. Ruxton, 2002. Living in Groups. Oxford University Press, Oxford.
Krause, J., J.-G. J. Godin & D. Brown, 1996. Phenotypic variability within and between fish shoals. Ecology 77: 1586–1591.
Kullmann, H., T. Thünken, S. A. Baldauf, T. C. M. Bakker & J. G. Frommen, 2008. Fish odour triggers conspecific attraction behaviour in an aquatic invertebrate. Biology Letters 4: 458–460.
Landeau, L. & J. Terborgh, 1986. Oddity and the ‘confusion effect’ in predation. Animal Behaviour 34: 1372–1380.
Lefèvre, T., C. Lebarbenchon, M. Gauthier-Clerc, D. Missé, R. Poulin & F. Thomas, 2009. The ecological significance of manipulative parasites. Trends in Ecology and Evolution 24: 41–48.
Magurran, A. E., 1990. The adaptive significance of schooling as an anti-predator defence in fish. Annales Zoologici Fennici 27: 51–66.
Maynard, B. J., T. A. Wellnitz, N. Zanini, W. G. Wright & B. S. Dezfuli, 1998. Parasite-altered behavior in a crustacean intermediate host: field and laboratory studies. Journal of Parasitology 84: 1102–1106.
Mazzi, D. & T. C. M. Bakker, 2003. A predator’s dilemma: prey choice and parasite susceptibility in three-spined sticklebacks. Parasitology 126: 339–347.
McCahon, C. P., A. F. Brown & D. Pascoe, 1988. The effect of the acanthocephalan Pomphorhynchus laevis (Müller 1776) on the acute toxicity of cadmium to its intermediate host, the amphipod Gammarus pulex (L). Archives of Environmental Contamination and Toxicology 17: 239–243.
Médoc, V. & J. N. Beisel, 2008. An Acanthocephalan parasite boosts the escape performance of its intermediate host facing non-host predators. Parasitology 135: 1977–1984.
Médoc, V. & J. N. Beisel, 2009. Field evidence for non-host predator avoidance in a manipulated amphipod. Naturwissenschaften 96: 513–523.
Médoc, V., L. Bollache & J. N. Beisel, 2006. Host manipulation of a freshwater crustacean (Gammarus roeseli) by an acanthocephalan parasite (Polymorphus minutus) in a biological invasion context. International Journal for Parasitology 36: 1351–1358.
Médoc, V., T. Rigaud, L. Bollache & J. N. Beisel, 2009. A manipulative parasite increasing an antipredator response decreases its vulnerability to a nonhost predator. Animal Behaviour 77: 1235–1241.
Moore, J., 2002. Parasites and the Behaviour of Animals. Oxford University Press, Oxford.
Perrot-Minnot, M. J., N. Kaldonski & F. Cezilly, 2007. Increased susceptibility to predation and altered anti-predator behaviour in an acanthocephalan-infected amphipod. International Journal for Parasitology 37: 645–651.
Pitcher, T. J. & J. K. Parrish, 1993. Functions of shoaling behaviour in teleosts. In Pitcher, T. J. (ed.), The Behaviour of Teleost Fishes. Croom Helm, London: 363–439.
Poulin, R., 1994. Meta-analysis of parasite-induced behavioural changes. Animal Behaviour 48: 137–146.
Poulton, M. J. & D. J. Thompson, 1987. The effects of the acanthocephalan parasite Pomphorhynchus laevis on mate choice in Gammarus pulex. Animal Behaviour 35: 1577–1579.
R Development Core Team, 2009. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.
Roberts, G., 1996. Why individual vigilance declines as group size increases. Animal Behaviour 51: 1077–1086.
Theodorakis, C. W., 1989. Size segregation and the effect of oddity on predation risk in minnow schools. Animal Behaviour 38: 496–502.
Thomas, F., S. Adamo & J. Moore, 2005. Parasitic manipulation: where are we and where should we go? Behavioural Processes 68: 185–199.
Ward, A. J. W., A. J. Duff, J. Krause & I. Barber, 2005. Shoaling behaviour of sticklebacks infected with the microsporidian parasite, Glugea anomala. Environmental Biology of Fishes 72: 155–160.
Wellnitz, T., L. Giari, B. Maynard & B. S. Dezfuli, 2003. A parasite spatially structures its host population. Oikos 100: 263–268.
Welty, J., 1934. Experiments in group behavior of fishes. Physiological Zoology 7: 85–128.
Wooster, D. E., 1998. Amphipod (Gammarus minus) responses to predators and predator impact on amphipod density. Oecologia 115: 253–259.
Acknowledgements
We thank Tobias Ottenheym for assistance in experiment 1. The ‘Bakker’ research-group is acknowledged for discussion. We thank the associated editor and the anonymous referees for their very useful comments on the manuscript. Kathrin Langen is acknowledged for genetic determination of the parasite. The Untere Landschaftsbehörde Bonn kindly allowed taking gammarids from the natural habitat. This study adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: S. Stendera
Rights and permissions
About this article
Cite this article
Thünken, T., Baldauf, S.A., Bersau, N. et al. Impact of olfactory non-host predator cues on aggregation behaviour and activity in Polymorphus minutus infected Gammarus pulex . Hydrobiologia 654, 137–145 (2010). https://doi.org/10.1007/s10750-010-0377-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0377-6