Abstract
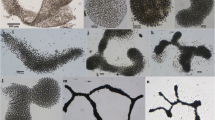
Recent advances in the taxonomy of cyanobacteria due to the utilization of molecular methods (such as 16S rRNA analysis) require a comparison of genetically identified items with their morphological expression. Morphological variability of seven monoclonal populations of the planktonic coccoid genus Chroococcus Nägeli, isolated mostly from reservoirs, fishponds, and littoral substrata from localities in the Czech and Slovak Republics, were studied experimentally under various combinations of nutrient concentration, light intensity, temperature, and water movement. Two cultivation media (WH-WC and BG11) commonly used in algal collections were applied in a liquid state. Of these, the WH medium was found to be more convenient for planktonic forms. Impacts of combined temperature and light gradients, concentration of P–PO4, and a stable versus shaken medium were found to stress different morphological modifications as a consequence of varied growth intensity and media convenience. Cell width was chosen as the parameter for testing changes in morphology; formation of mucilage and packets of cells were also taken into account. According to 16S rRNA gene analysis, the sequences of 10 strains (including seven studied in the experiments), which were assigned to the genus Chroococcus in the Culture Collection CCALA Třeboň (www.cas.ccala.cz) formed four distinct phylogenetic groups. While two of them showed no affiliation to the genus Chroococcus, two other groups proved the polyphyletic character of the genus. Apart from the group of typical species of the genus Chroococcus, a group of planktonic species could be distinguished, i.e., Chroococcus limneticus Lemmermann 1898 (Limnococcus), and was established as a new genus after recombination.





Similar content being viewed by others
References
Allen, M. M. & R. Y. Stanier, 1968. Growth and division of some unicellular blue-green algae. Journal of Genetics and Microbiology 51: 199–202.
Anand, N., 1988. Culture studies and taxonomy of blue-green algae—certain identification problems. Archiv für Hydrobiologie Supplement 80: 141–147.
Anonymous, 1996. Statistica for Windows [Computer program manual]. Statsoft, Tulsa.
Castenholz, R. W. & J. B. Waterbury, 1989. Cyanobacteria. In: Staley, J. T., M. P. Bryant, N. Pfennig & J. G. Holt (eds), Bergey’s Manual of Systematic Bacteriology, Vol. 3. Williams & Wilkins, Baltimore: 1710–1727.
Flechtner, V. R., S. L. Boyer & J. R. Johansen, 2002. Spirirestis rafaelensis gen. et sp. nov. (Cyanophyceae), a new cyanobacterial genus from arid soils. Nova Hedwigia 74: 1–24.
Geitler, L., 1932. Cyanophyceae. Rabenhorst’s Kryptogamenflora 14: 1–1196.
Greuter, W., H. M. Burdet, W. G. Chaloner, V. Demoulin, R. Grolle, D. L. Hawks-Worth, D. H. Nicolson, P. C. Silva, F. A. Stafleu, E. G. Wos & J. McNeil, 1988. International Code of Botanical Nomenclature. Koeltz Scientific Books, Königstein: 328 pp.
Gugger, M., C. Lyra, P. Hendriksen, A. Couté, J. F. Humbert & K. Sivonen, 2002. Phylogenetic comparison of the cyanobacterial genera Anabaena and Aphanizomenon. International Journal of Systematic and Evolutionary Microbiology 52: 1–14.
Guillard, R. R. & C. Lorenzen, 1972. Yellow green algae with chlorophyllide c. Journal of Phycology 8: 10–14.
Hensyl, W. R. (ed.), 1994. Bergey’s Manual of Determinative Bacteriology, 9th ed.
Jezberová, J. & J. Komárková, 2007. Morphological transformation in a freshwater Cyanobium sp. induced by grazers. Environmental Microbiology 9: 1858–1862.
Komárek, J., 1969. Morphological variability of “Anacystis nidulans”. Annual Report of the Laboratory of Algology for the year 1968, Trebon: 19–24.
Komárek, J. & K. Anagnostidis, 1998. Cyanoprokaryota. 1. Teil: Chroococcales. In Ettl, H., G. Gärtner, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa. Gustav Fischer Verl, Jena: 548 pp.
Komárková, J., M. A. Mugnai, C. Sili, O. Komárek & S. Turicchia, 2005. Stable morphospecies within the 16S rRNA monophyletic genus Microcystis (Kützing) Küetzing. Algological Studies 117 (Cyanobacterial Research 6): 279–295.
Kvíderová, J. & J. Lukavský, 2001. A new unit for crossed gradients of temperature and light. In: Elster et al. (ed), Algae and Extreme Environments. Proc. Int. Conf. 11–16. Sept., Třeboň, CZ. Nova Hedwigia, Beihefte 123: 539–548.
Li, R., M. Watanabe & M. M. Watanabe, 2000. Taxonomic studies on planktic species of Anabaena based on morphological characteristics in cultured strains. Hydrobiologia 438: 117–138.
Lukavský, J., 1982. Cultivation of chlorococcal algae in crossed gradients of temperature and light. Archiv für Hydrobiologie, Algological Studies 29: 517–528.
Palinska, K., W. Liesack, E. Rhiel & W. E. Krumbein, 1996. Phenotype variability of identical genotypes: the need for a combined approach in cyanobacterial taxonomy demonstrated on Merismopedia-like isolates. Archives of Microbiology 166: 224–233.
Pollingher, U., 1991. A long-term study of Chroococcus population in Lake Kinneret (Israel). Algological Studies 64: 377–383.
Rajaniemi-Wacklin, P., A. Rantala, M. A. Mugnai, S. Turicchia, S. Ventura, J. Komárková, L. Lepistö & K. Sivonen, 2005. Correspondence between phylogeny and morphology of Snowella spp. and Woronichinia naegeliana, Cyanobacteria, commonly occurring in lakes. Journal of Phycology 42: 226–232.
Richert, L., S. Golubic, R. Le Guedes, A. Herve & C. Payri, 2006. Cyanobacterial populations that build ‘kopara’ microbial mats in Rangiroa, Tuamotu Archipelago, French Polynesia. European Journal of Phycology 41: 259–279.
Rudi, K., O. M. Skulberg & K. S. Jacobsen, 1998. Evolution of Cyanobacteria by exchange of genetic material among phyletically related strains. Journal of Bacteriology 180: 3453–3461.
Saitou, N. & M. Nei, 1987. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Molecular and Biological Evolution 4: 406–425.
Stanier, R. Y., R. Kurisawa, R. Mandel & G. Cohen-Bazire, 1971. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriological Review 35: 171–205.
Staub, R., 1961. Ernährungsphysiologische Untersuchungen an der planktonischen Blaualge Oscillatoria rubescens DC. Schweizerische Zeitschrift für Hydrologie 23: 82–198.
Tamura, K., J. Dudley, M. Nei & S. Kumar, 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular and Biological Evolution 24: 1596–1599.
Tandeau de Marsac, N. & J. Houmard, 1993. Adaptation of cyanobacteria to environmental stimuli: new steps towards molecular mechanisms. FEMS Microbiology Letters 104: 119–189.
Taton, A., S. Grubisic, E. Brambilla, R. De Wit & A. Wilmotte, 2003. Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo dry valleys, Antarctica): a morphological and molecular approach. Applied and Environmental Microbiology 69: 5157–5169.
Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. I. Krichevsky, W. E. C. Moore, R. G. E. Murray, E. Stackebrand, M. P. Starr & H. G. Trüper, 1987. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. International Journal of Systematic and Evolutionary Microbiology 37: 463–464.
Weisse, T., 1993. Dynamics of autotrophic Picoplankton in marine and freshwater ecosystems. Advances in Microbial Ecology 13: 327–370.
Wilmotte, A., 1988. Growth and morphological variability of six strains of Phormidium cf. ectocarpi Gomont (Cyanophyceae) cultivated under different temperatures and light intensities. Algological Studies 50–53: 35–46.
Wyman, M. & P. Fay, 1997. Acclimation to the natural light climate. In Fay, P. & C. Van Baalen (eds), The Cyanobacteria. Elsevier Science Publishers B.V. (Biomedical Division), Amsterdam: 347–376.
Zapomělová, E., D. Hisem, K. Řeháková, P. Hrouzek, J. Jezberová, J. Komárková, J. Korelusová & P. Znachor, 2008a. Experimental comparison of phenotypical plasticity and growth demands of two strains from the Anabaena circinalis/A. crassa complex (cyanobacteria). Journal of Plankton Research 30: 1257–1269.
Zapomělová, E., P. Hrouzek, K. Řeháková, M. Šabacká, M. Stibal, L. Caisová, J. Komárková & A. Lukešová, 2008b. Morphological variability in selected heterocystous cyanobacterial strains as a response to varied temperature, light intensity and medium composition. Folia Microbiologica 53: 333–340.
Zapomělová, E., K. Řeháková, J. Jezberová & J. Komárková, 2009. Polyphasic characterization of eight planktonic Anabaena strains (cyanobacteria) with reference to the variability of 61 Anabaena populations observed in the field. Hydrobiologia (this volume).
Acknowledgments
The authors wish to thank J. Komárek for valuable discussions, F. Hindák, L. Kováčik, and a staff of CCALA in Třeboň who supplied and maintained strains for the culture collection and consequently for our experiments, and M. Kupková from the Hydrobiological Institute AS CR for technical help. Financial support was provided by the following projects: 206/09/0309 and 206/07/0917 by Grant Agency of Czech Republic and AV0Z60170517 awarded to HBI AS CR. This study was presented as a contributed article at the Bat Sheva de Rothschild seminar on Phytoplankton in the Physical Environment—The 15th Workshop of the International Association of Phytoplankton Taxonomy and Ecology (IAP)—held in Ramot, Israel, November 23–30, 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: T. Zohary, J. Padisák & L. Naselli-Flores / Phytoplankton in the Physical Environment: Papers from the 15th Workshop of the International Association for Phytoplankton Taxonomy and Ecology (IAP), held at the Ramot Holiday Resort on the Golan Heights, Israel, 23–30 November 2008
Rights and permissions
About this article
Cite this article
Komárková, J., Jezberová, J., Komárek, O. et al. Variability of Chroococcus (Cyanobacteria) morphospecies with regard to phylogenetic relationships. Hydrobiologia 639, 69–83 (2010). https://doi.org/10.1007/s10750-009-0015-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-0015-3