Abstract
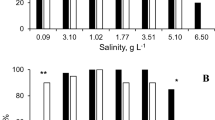
Suspended particles are abiotic factors that can affect the abundance of cladocerans such as daphnids. Ultra-oligotrophic Lake Brienz, situated in the front ranges of the Swiss Alps, is dominated by two major inflows that annually transport over 300,000 tons of suspended glacial material into the lake. A laboratory flow-through experiment was performed to test whether these suspended particles have an influence on the fitness of Daphnia hyalina from Lake Brienz, measured as body size, fecundity and juvenile growth rate, especially when they are simultaneously exposed to low food concentrations. Our results show that the concentration of suspended particles present in Lake Brienz does not reduce the fitness of daphnids, even at very low food concentration. In fact, a low concentration of suspended particles increased the fitness. Reduction of fitness could only be observed at a suspended particle concentration of over 25 mg l−1, a level that has never been recorded in Lake Brienz.




Similar content being viewed by others
References
Adalsteinsson, H., 1979. Zooplankton and its relation to available food in Lake Myvatn. Oikos 32: 162–194.
Arruda, J. A., G. R. Marzolf & R. T. Faulk, 1983. The role of suspended sediments in the nutrition of zooplankton in turbid reservoirs. Ecology 64: 1225–1235.
Bozelli, R. L., 1998. Influences of suspended inorganic matter on carbon ingestion and incorporation rates of two tropical cladocerans, Diaphanosoma birgei and Moina minuta. Archiv für Hydrobiologie 142: 451–465.
Carvalho, M. L., 1984. Influence of predation by fish and water turbidity on a Daphnia-Gessneri population in an amazonian floodplain lake, Brazil. Hydrobiologia 113: 243–247.
Chiou, C. T., L. J. Peters & V. H. Freed, 1979. Physical concept of soil-water equilibria for non-ionic organic-compounds. Science 206: 831–832.
Demott, W. R., 1982. Feeding selectivities and relative ingestion rates of Daphnia and Bosmina. Limnology and Oceanography 27: 518–527.
Finger, D., M. Schmid & A. Wüest, 2006. Effects of upstream hydropower operation on riverine particle transport and turbidity in downstream lakes. Water Resources Research 42: W08429, doi:10.1029/2005WR004751.
G-Toth, L., K. V-Balogh & N. P. Zankai, 1986. Significance and degreee of abioseston consumption in the filter-feeder Daphnia galeata Sars am. Richard (Cladocera) in Lake Balaton. Archiv für Hydrobiologie 106: 45–60.
Gionfriddo, J. P. & L. B. Best, 1996. Grit-use patterns in North American birds: The influence of diet, body size, and gender. Wilson Bulletin 108: 685–696.
Gliwicz, M. Z., 1986. Suspended clay concentration controlled by filter-feeding zooplankton in a tropical reservoir. Nature 323: 330–332.
Hart, R. C., 1986. Zooplankton abundance, community structure and dynamics in relation to inorganic turbidity, and their implications for a potential fishery in subtropical Lake Le-Roux, South-Africa. Freshwater Biology 16: 351–371.
Hart, R. C., 1987. Population-dynamics and production of 5 crustacean zooplankters in a subtropical reservoir during years of contrasting turbidity. Freshwater Biology 18: 287–318.
Hart, R. C., 1992. Experimental studies of food and suspended sediment effects on growth and reproduction of 6 planktonic cladocerans. Journal of Plankton Research 14: 1425–1448.
Jewson, D. H. & J. A. Taylor, 1978. Influence of turbidity on net phytoplankton photosynthesis in some Irish lakes. Freshwater Biology 8: 573–584.
Kirk, K. L., 1991a. Inorganic particles alter competition in grazing plankton—the role of selective feeding. Ecology 72: 915–923.
Kirk, K. L., 1991b. Suspended clay reduces Daphnia feeding rate—behavioral mechanisms. Freshwater Biology 25: 357–365.
Kirk, K. L., 1992. Effects of suspended clay on Daphnia body growth and fitness. Freshwater Biology 28: 103–109.
Kirk, K. L. & J. J. Gilbert, 1990. Suspended clay and the population dynamics of planktonic rotifers and cladocerans. Ecology 71: 1741–1755.
Koenings, J. P., R. D. Burkett & J. M. Edmundson, 1990. The exclusion of limnetic cladocera from turbid glacier-meltwater lakes. Ecology 71: 57–67.
Krause-Jensen, D. & K. Sand-Jensen, 1998. Light attenuation and photosynthesis of aquatic plant communities. Limnology and Oceanography 43: 396–407.
Lampert, W., 1977. Studies on the carbon balance of Daphnia pulex as related to environmental conditions—IV. Determination of the “threshold” concentration as a factor controlling the abundance of zooplankton species. Archiv für Hydrobiologie/Supplement 48: 361–368.
Lampert, W., 1978. A field study on the dependence of the fecundity of Daphnia spec. on food concentration. Oecologia 36: 363–369.
Lampert, W., 1989. The adaptive significance of diel vertical migration of zooplankton. Functional Ecology 3: 21–28.
Lampert, W. & I. Trubetskova, 1996. Juvenile growth rate as a measure of fitness in Daphnia. Functional Ecology 10: 631–635.
Lampert, W. & U. Sommer, 1999, Limnoökologie. Georg Thieme Verlag. Stuttgart, New York.
Maedamartinez, A. M., H. Obregonbarboza & H. J. Dumont, 1995. Laboratory culture of fairy shrimps using bakers-yeast as basic food in a flow-through system. Hydrobiologia 298: 141–157.
Manca, M. & D. Ruggiu, 1998. Consequences of pelagic food-web changes during a long-term lake oligotrophication process. Limnology and Oceanography 43: 1368–1373.
McCabe, G. D. & W. J. O’Brien, 1983. The effects of suspended silt on feeding and reproduction of Daphnia pulex. American Midland Naturalist 110: 324–337.
McCarthy, J. F., 1983. Role of particulate organic-matter in decreasing accumulation of polynuclear aromatic-hydrocarbons by Daphnia magna. Archives of Environmental Contamination and Toxicology 12: 559–568.
Müller-Navarra, D. & W. Lampert, 1996. Seasonal patterns of food limitation in Daphnia galeata: separating food quantity and food quality effects. Journal of Plankton Research 18: 1137–1157.
Müller, B., R. Stierli & A. Wüest, 2006. Phosphate adsorption by mineral weathering particles in oligotrophic waters of high particle content. Water Resources Research 42: W10414, doi:10.1029/2005WR004778.
Paloheimo, I. E., 1974. Calculation of instantaneous birth rate. Limnology and Oceanography 19: 692–694.
Rellstab, C., V. Maurer, M. Zeh, H. Bürgi & P. Spaak, 2007. Temporary collapse of the Daphnia population in turbid and ultra-oligotrophic Lake Brienz. Aquatic Sciences 69: 257–270.
Robinson, M., 1957. The effects of suspended materials on the reproductive rate of Daphnia magna. Publications of the Institute of Marine Science 4: 265–277.
Scavia, D., G. L. Fahnenstiel, J. A. Davis & R. G. Kreis, 1984. Small-scale nutrient patchiness—some consequences and a new encounter mechanism. Limnology and Oceanography 29: 785–793.
Sturm, M., 1976. Die Oberflächensedimente des Brienzersees. Eclogae Geologicae Helvetiae 69: 111–123.
Sturm, M. & A. Matter, 1978. Turbidites and varves in Lake Brienz (Switzerland); deposition of clastic detritus by density currents. Special Publication of the International Association of Sedimentologists 2: 147–168.
Vanderploeg, H. A., B. J. Eadie, J. R. Liebig & S. J. Tarapchak, 1987. Contribution of calcite to the particle-size spectrum of Lake Michigan seston and its interactions with the plankton. Canadian Journal of Fisheries and Aquatic Sciences 44: 1898–1914.
Vinyard, G. L. & W. J. O’Brien, 1976. Effects of light and turbidity on reactive distance of bluegill (Lepomis macrochirus). Journal of the Fisheries Research Board of Canada 33: 2845–2849.
Zettler, E. R. & J. C. H. Carter, 1986. Zooplankton community and species responses to a natural turbidity gradient in Lake Temiskaming, Ontario Quebec. Canadian Journal of Fisheries and Aquatic Sciences 43: 665–673.
Zurek, R., 1980. Effect of suspended materials on zooplankton—1. Natural environments. Acto Hydrobiologica 22: 449–471.
Zurek, R., 1982. Effect of suspended materials on zooplankton—2. Laboratory investigations of Daphnia hyalina Leydig. Acto Hydrobiologica 24: 233–251.
Acknowledgements
Sabine Rellstab, Christine Dambone, Piotr Madej and Christoph Tellenbach helped set up and perform the experiment. Alois Zwyssig, David Finger, Mike Sturm and Erwin Grieder assisted in sampling or processing the sediment core. Richard Illi measured POC concentrations of the water samples. Bastian Bommer performed the particle size distribution measurements. Barbara Keller, Justyna Wolinska, Wayne Wurtsbaugh and two anonymous reviewers helped to improve earlier versions of this manuscript. Lisa Shama provided statistical advice. We are very grateful for their help. This study is part of the “Lake Brienz Project” and was financed by Eawag, the canton of Berne, KWO Grimselpower, BUWAL and regional communities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editor: Piet Spaak
Cladocera: Proceedings of the 7th International Symposium on Cladocera
Rights and permissions
About this article
Cite this article
Rellstab, C., Spaak, P. Starving with a full gut? Effect of suspended particles on the fitness of Daphnia hyalina . Hydrobiologia 594, 131–139 (2007). https://doi.org/10.1007/s10750-007-9089-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9089-y