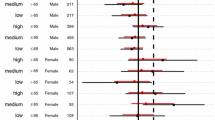
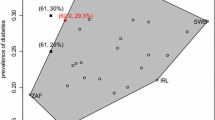
Abstract
Subgroup analysis is a frequently used tool for evaluating heterogeneity of treatment effect and heterogeneity in treatment harm across observed baseline patient characteristics. While treatment efficacy and adverse event measures are often reported separately for each subgroup, analyzing their within-subgroup joint distribution is critical for better informed patient decision-making. In this paper, we describe Bayesian models for performing a subgroup analysis to compare the joint occurrence of a primary endpoint and an adverse event between two treatment arms. Our approach emphasizes estimation of heterogeneity in this joint distribution across subgroups, and our approach directly accommodates subgroups with small numbers of observed primary and adverse event combinations. In addition, we describe several ways in which our models may be used to generate interpretable summary measures of benefit-risk tradeoffs for each subgroup. The methods described here are illustrated throughout using a large cardiovascular trial (\(N = 9361\)) investigating the efficacy of an intervention for reducing systolic blood pressure to a lower-than-usual target.






Similar content being viewed by others
References
Boughorbel, S., Jarray, F., El-Anbari, M.: Optimal classifier for imbalanced data using Matthews correlation coefficient metric. PloS ONE 12(6), e0177678 (2017)
Carlin, B.P., Louis, T.A.: Bayesian Methods for Data Analysis. Chapman and Hall/CRC, Boca Raton (2009)
Carpenter, B., Gelman, A., Hoffman, M., Lee, D., Goodrich, B., Betancourt, M., Brubaker, M., Guo, J., Li, P., Riddell, A.: Stan: a probabilistic programming language. J. Stat. Softw. 76(1), 1–32 (2017)
Chuang-Stein, C.: A new proposal for benefit-less-risk analysis in clinical trials. Control Clin. Trials 15, 30–43 (1994)
Claggett, B., Tian, L., Castagno, D., Wei, L.-J.: Treatment selections using risk–benefit profiles based on data from comparative randomized clinical trials with multiple endpoints. Biostatistics 16(1), 60–72 (2015)
Dixon, D.O., Simon, R.: Bayesian subset analysis. Biometrics 47, 871–881 (1991)
Efron, B., Morris, C.: Stein’s estimation rule and its competitors—an empirical Bayes approach. J. Am. Stat. Assoc. 68(3), 117–130 (1973)
Evans, S.R., Follmann, D.: Using outcomes to analyze patients rather than patients to analyze outcomes: a step toward pragmatism in benefit:risk evaluation. Stat. Biopharm. Res. 8(4), 386–393 (2016)
Follmann, D.: Regression analysis based on pairwise ordering of patients clinical histories. Stat. Med. 21(22), 3353–3367 (2002)
Gelber, R.D., Gelman, R.S., Goldhirsch, A.: A quality-of-life oriented endpoint for comparing treatments. Biometrics 45(3), 781–795 (1989)
Gelman, A., Carlin, J., Stern, H., Dunson, D., Vehtari, A., Rubin, D.: Bayesian Data Analysis (Chapman & Hall/CRC Texts in Statistical Science), 3rd edn. Chapman and Hall/CRC, London (2014)
Henderson, N.C., Louis, T.A., Wang, C., Varadhan, R.: Bayesian analysis of heterogeneous treatment effects for patient-centered outcomes research. Health Serv. Outcomes Res. Methodol. 16, 213–233 (2016)
Holford, T.R.: The analysis of rates and of survivorship using log-linear models. Biometrics 36(2), 299–305 (1980)
Jones, H.E., Ohlssen, D.I., Neuenschwander, B., Racine, A., Branson, M.: Bayesian models for subgroup analysis in clinical trials. Clin. Trials 8, 129–143 (2011)
Meng, X.L.: Posterior predictive p values. Ann. Stat. 23(3), 1142–1160 (1994)
Padmanabhan, S.K., Berry, S., Dragalin, V., Krams, M.: A Bayesian dose-finding design adapting to efficacy and tolerability response. J. Biopharm. Stat. 22(2), 276–293 (2012)
Royston, P., Parmar, M.K.: Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med. Res. Methodol. 13(1), 152 (2013)
Spiegelhalter, D.J., Best, N.G., van der Carlin, B.P., Linde, A.: Bayesian measures of model complexity and fit (with discussion). J. R. Stat. Soc. B 64(4), 583–639 (2002)
The SPRINT Research Group: A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 373(22), 2103–2116 (2015)
Watanabe, S.: Asymptotic equivalence of Bayes cross validation and widely applicable information criterion in singular learning theory. J. Mach. Learn. Res. 11, 3571–3594 (2010)
Funding
The authors were supported by the National Institute of Health’s (NIH) National Center for the Advancement of Translational Sciences (NCATS) through the Grant Number UL1TR001079-04S1, and by the NIH Grant Number P30CA006973.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Henderson, N.C., Varadhan, R. Bayesian bivariate subgroup analysis for risk–benefit evaluation. Health Serv Outcomes Res Method 18, 244–264 (2018). https://doi.org/10.1007/s10742-018-0188-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10742-018-0188-1