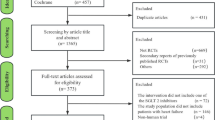
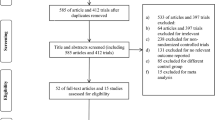
Abstract
The recently published randomized trials (RCTs) evaluating the effect of Sodium-glucose cotransporter-2 inhibitors (SGLT2i) in heart failure with mildly reduced (HFmrEF) or preserved ejection fraction (HFpEF) led researchers to perform a plethora of systematic reviews (SRs), often providing contradictory conclusions. This overview of reviews was aimed at summarizing the evidence of these SRs, quantifying the overlap, re-analyzing the evidence in case new studies that were identified, and mapping knowledge gaps. Literature search was conducted through Medline, Scopus, and Cochrane until March 22, 2023. Overall, 36 SRs synthesizing results from 18 RCTs were identified. A substantial overlap was identified among the SRs synthesizing large heart failure or cardiovascular outcome trials (CVOTs). Regarding the composite outcome of cardiovascular (CV) mortality or hospitalization for heart failure (HHF), all authors reported a significant favorable effect. A beneficial effect was also noted for CV and all-cause mortality, albeit not significant. Our meta-analysis demonstrated a significant improvement in health-related quality-of-life (HRQoL) as assessed by the Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OSS, MD = 1.97, p < 0.001), Total Symptom Score (KCCQ-TSS, MD = 2.29, p < 0.001), Clinical Summary Score (KCCQ-CSS, MD = 1.59, p < 0.001), and the 6-min walking distance (MD = 10.78 m, p = 0.032). Regarding safety, SGLT2i were associated with a significantly lower risk of serious adverse events compared to placebo (RR = 0.94, p = 0.002). The use of SGLT2i in HFpEF is both efficient and safe. Further research is required to clarify the impact of SGTL2i on different subphenotypes of HFpEF and the cardiorespiratory capacity of these patients.



Similar content being viewed by others
Data availability
All data extracted and analyzed in this study can be accessed here (https://osf.io/p6a9w/).
Abbreviations
- CV:
-
Cardiovascular
- HHF:
-
Hospitalization for heart failure
- HFmrEF:
-
Heart failure with mildly reduced ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- SGLT2i:
-
Sodium-glucose cotransporter-2 inhibitors
- KCCQ-TSS:
-
Kansas City Cardiomyopathy Questionnaire Total Symptom Score
- KCCQ-CSS:
-
Clinical Summary Score
- KCCQ-OSS:
-
Overall Summary Score
- KCCQ-PL:
-
Physical Limitation
- 6MWD:
-
6-Minute walk distance
References
Dunlay SM, Roger VL, Redfield MM (2017) Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 14:591–602. https://doi.org/10.1038/nrcardio.2017.65
Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP et al (2012) Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 126:65–75. https://doi.org/10.1161/CIRCULATIONAHA.111.080770/-/DC1
Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP et al (2018) Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 391:572–580. https://doi.org/10.1016/S0140-6736(17)32520-5
Pfeffer MA, Shah AM, Borlaug BA (2019) Heart failure with preserved ejection fraction in perspective. Circ Res 124:1598–1617. https://doi.org/10.1161/CIRCRESAHA.119.313572
Bui AL, Horwich TB, Fonarow GC (2010) Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011 81:8:30–41. https://doi.org/10.1038/nrcardio.2010.165
Marwick TH, Ritchie R, Shaw JE, Kaye D (2018) Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol 71:339–351. https://doi.org/10.1016/J.JACC.2017.11.019
McHugh K, DeVore AD, Wu J, Matsouaka RA, Fonarow GC, Heidenreich PA et al (2019) Heart failure with preserved ejection fraction and diabetes: JACC State-of-the-Art Review. J Am Coll Cardiol 73:602–611. https://doi.org/10.1016/J.JACC.2018.11.033
Parasuraman SK, Loudon BL, Lowery C, Cameron D, Singh S, Schwarz K et al (2019) Diastolic ventricular interaction in heart failure with preserved ejection fraction. J Am Heart Assoc 8. https://doi.org/10.1161/JAHA.118.010114
McDonagh TA, Metra M, Adamo M, Baumbach A, Böhm M, Burri H et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failuredeveloped by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 42:3599–3726. https://doi.org/10.1093/EURHEARTJ/EHAB368
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM et al (2022) 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145:E895-1032. https://doi.org/10.1161/CIR.0000000000001063
Ahmad Y, Madhavan MV, Stone GW, Francis DP, Makkar R, Bhatt DL et al (2022) Sodium-glucose cotransporter 2 inhibitors in patients with heart failure: a systematic review and meta-analysis of randomized trials. Eur Hear J - Qual Care Clin Outcomes 8:383–390. https://doi.org/10.1093/ehjqcco/qcab072
Tsampasian V, Elghazaly H, Chattopadhyay R, Ali O, Corballis N, Chousou PA et al (2022) Sodium glucose co-transporter 2 inhibitors in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Prev Cardiol 29:e227–e229. https://doi.org/10.1093/eurjpc/zwab189
Cao Y, Li P, Li Y, Han Y (2022) Sodium-glucose cotransporter-2 inhibitors in heart failure: an updated meta-analysis. ESC Hear Fail 9:1942–1953. https://doi.org/10.1002/ehf2.13905
Requena-Ibanez JA, Santos-Gallego CG, Zafar MU, Badimon JJ (2022) SGLT2-inhibitors on HFpEF patients. Role of ejection fraction. Cardiovasc Drugs Ther 1–8. https://doi.org/10.1007/S10557-022-07371-7/METRICS
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. 2021;385:1451–61. https://doi.org/10.1056/NEJMOA2107038.
Spertus JA, Birmingham MC, Nassif M, Damaraju CV, Abbate A, Butler J et al (2022) The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med 284 2022;28:809–13. https://doi.org/10.1038/s41591-022-01703-8
Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F et al (2021) The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med 27:1954–60. https://doi.org/10.1038/s41591-021-01536-x
Abraham WT, Lindenfeld JA, Ponikowski P, Agostoni P, Butler J, Desai AS et al (2021) Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J 42:700–710. https://doi.org/10.1093/EURHEARTJ/EHAA943
Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM et al (2019) Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 139:2528–2536. https://doi.org/10.1161/CIRCULATIONAHA.119.040130
Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S et al (2020) Efficacy of ertugliflozin on heart failure–related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease. Circulation 142:2205–2215. https://doi.org/10.1161/CIRCULATIONAHA.120.050255
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M et al (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385:1451–1461. https://doi.org/10.1056/NEJMOA2107038/SUPPL_FILE/NEJMOA2107038_DATA-SHARING.PDF
Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK et al (2021) Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 384:129–139
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK et al (2021) Sotagliflozin in patients with diabetes and recent worsening heart failure 384:117–128
Solomon SD, McMurray JJV, Claggett B, Boer RA de, DeMets D, Hernandez AF et al (2022) Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction 387:1089–98. https://doi.org/10.1056/NEJMOA2206286
Hoffmann F, Allers K, Rombey T, Helbach J, Hoffmann A, Mathes T et al (2021) Nearly 80 systematic reviews were published each day: observational study on trends in epidemiology and reporting over the years 2000–2019. J Clin Epidemiol 138:1–11. https://doi.org/10.1016/J.JCLINEPI.2021.05.022
Pollock M, Fernandes RM, Becker LA, Pieper D HL (2022) Chapter V: overviews of reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from. n.d.
Hartling L, Vandermeer B, Fernandes RM (2014) Systematic reviews, overviews of reviews and comparative effectiveness reviews: a discussion of approaches to knowledge synthesis. Evid Based Child Health 9:486–494. https://doi.org/10.1002/EBCH.1968
Cochrane handbook for systematic reviews of interventions | Cochrane Training (n.d) https://training.cochrane.org/handbook/current (Accessed January 17, 2023).
Gates M, Gates A, Pieper D, Fernandes R, Tricco A, Moher D et al (2022) Reporting guideline for overviews of reviews of healthcare interventions: the Preferred Reporting Items for Overviews of Reviews (PRIOR) statement https://doi.org/10.31222/OSF.IO/82WAU
Bougioukas KI, Liakos A, Tsapas A, Ntzani E, Haidich AB (2018) Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol 93:9–24. https://doi.org/10.1016/J.JCLINEPI.2017.10.002
Bougioukas KI, Bouras E, Apostolidou-Kiouti F, Kokkali S, Arvanitidou M, Haidich AB (2019) Reporting guidelines on how to write a complete and transparent abstract for overviews of systematic reviews of health care interventions. J Clin Epidemiol 106:70–79. https://doi.org/10.1016/J.JCLINEPI.2018.10.005
Haddaway NR, Grainger MJ, Gray CT (2021) citationchaser: an R package and Shiny app for forward and backward citations chasing in academic searching. https://doi.org/10.5281/ZENODO.4543513
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J et al (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:1–9. https://doi.org/10.1136/bmj.j4008
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366. https://doi.org/10.1136/BMJ.L4898
Bougioukas KI, Diakonidis T, Mavromanoli AC, Haidich A-B (2022) ccaR: a package for assessing primary study overlap across systematic reviews in overviews. Res Synth Methods. https://doi.org/10.1002/JRSM.1610
Pieper D, Antoine SL, Mathes T, Neugebauer EAM, Eikermann M (2014) Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol 67:368–375. https://doi.org/10.1016/J.JCLINEPI.2013.11.007
GRADEpro (n.d) https://www.gradepro.org/ (Accessed March 1, 2023)
Al-Abdouh A, Mhanna M, Barbarawi M, Abusnina W, Gupta VA (2022) A meta-analysis of the sodium-glucose cotransporter 2 inhibitors in patients with heart failure and preserved ejection fraction. Am J Cardiol 164:138–141. https://doi.org/10.1016/j.amjcard.2021.10.017
Bazoukis G, Papadatos SS, Thomopoulos C, Tse G, Cheilidis S, Tsioufis K et al (2021) Impact of SGLT2 inhibitors on major clinical events and safety outcomes in heart failure patients: a meta-analysis of randomized clinical trials. J Geriatr Cardiol 18:783–95. https://doi.org/10.11909/j.issn.1671-5411.2021.10.003
Bhalla S, AlQabandi Y, Nandula SA, Boddepalli CS, Gutlapalli SD, Lavu VK et al (2022) Potential benefits of sodium-glucose transporter-2 inhibitors in the symptomatic and functional status of patients with heart failure: a systematic review and meta-analysis. Cureus 14:e29579. https://doi.org/10.7759/cureus.29579.
Butler J, Usman MS, Khan MS, Greene SJ, Friede T, Vaduganathan M et al (2020) Efficacy and safety of SGLT2 inhibitors in heart failure: systematic review and meta-analysis. ESC Hear Fail 7:3298–3309. https://doi.org/10.1002/EHF2.13169
Cardoso R, Graffunder FP, Ternes CMP, Fernandes A, Rocha A V, Fernandes G et al (2021) SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: a systematic review and meta-analysis. E Clinical Medicine 36. https://doi.org/10.1016/j.eclinm.2021.100933.
Chen H-B, Yang Y-L, Meng R-S, Liu X-W (2023) Indirect comparison of SGLT2 inhibitors in patients with established heart failure: evidence based on Bayesian methods. ESC Hear Fail. https://doi.org/10.1002/ehf2.14297
Chen X, Wang L, Li H, Huang W, Huang S, Zhao L et al (2022) Clinical benefit of sodium-glucose transport protein-2 inhibitors in patients with heart failure: an updated meta-analysis and trial sequential analysis. Front Cardiovasc Med 9. https://doi.org/10.3389/fcvm.2022.1067806
Fukuta H, Hagiwara H, Kamiya T (2022) Sodium-glucose cotransporter 2 inhibitors in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. IJC Hear Vasc 42. https://doi.org/10.1016/j.ijcha.2022.101103
Gager GM, Gelbenegger G, Jilma B, Lewinski D V, Sourij H, Eyileten C et al (2021) Cardiovascular outcome in patients treated with sglt2 inhibitors for heart failure: a meta-analysis. Front Cardiovasc Med 8:778284. https://doi.org/10.3389/fcvm.2021.691907
Ismayl M, Abbasi MA, Al-Abcha A, El-Am E, Lundgren S, Goldsweig AM et al (2023) Sodium-glucose cotransporter-2 inhibitors in heart failure with mildly reduced or preserved ejection fraction: a systematic review and meta-analysis of randomized controlled trials. Curr Probl Cardiol 48. https://doi.org/10.1016/j.cpcardiol.2023.101597
Ji P-J, Zhang Z-Y, Yan Q, Cao H-L, Zhao Y-J, Yang B et al (2023) The cardiovascular effects of SGLT2 inhibitors, RAS inhibitors, and ARN inhibitors in heart failure. ESC Hear Fail. https://doi.org/10.1002/ehf2.14298
Lin Y, Cai Z, Yuan J, Liu H, Pang X, Chen Q et al (2022) Effect of pharmacological treatment on outcomes of heart failure with preserved ejection fraction: an updated systematic review and network meta-analysis of randomized controlled trials. Cardiovasc Diabetol 21. https://doi.org/10.1186/s12933-022-01679-2.
Lu Y, Li F, Fan Y, Yang Y, Chen M, Xi J (2021) Effect of SGLT-2 inhibitors on cardiovascular outcomes in heart failure patients: a meta-analysis of randomized controlled trials. Eur J Intern Med 87:20–28. https://doi.org/10.1016/j.ejim.2021.03.020
Norre T, Grimm D, Simonsen U (2022) Sacubitril/valsartan, sodium-glucose cotransporter 2 inhibitors and vericiguat for congestive heart failure therapy. Basic Clin Pharmacol Toxicol 130:425–438. https://doi.org/10.1111/bcpt.13714
Pandey AK, Dhingra NK, Hibino M, Gupta V, Verma S (2022) Sodium-glucosecotransporter 2 inhibitors in heart failure with reduced or preserved ejectionfraction: a meta-analysis. ESC Hear Fail 9:942–946. https://doi.org/10.1002/ehf2.13805
Patoulias D, Michailidis T, Dimosiari A, Kassimis G, Fragakis N (2022) Meta-analysis addressing the impact of sodium-glucose Co-transporter-2 inhibitors on the risk for atrial fibrillation among individuals with heart failure with preserved ejection fraction. Int J Cardiol Cardiovasc Risk Prev 15. https://doi.org/10.1016/j.ijcrp.2022.200161
Qiu M, Ding L-L, Zhou1 H-R (2021) Factors affecting the efficacy of SGLT2is on heart failure events: a meta-analysis based on cardiovascular outcome trials. Cardiovasc Diagn Ther 11:699–706. https://doi.org/10.21037/cdt-20-984.
Qiu M, Zhao L-M (2021) Commentary: Cardiovascular outcome in patients treated with SGLT2 inhibitors for heart failure: a meta-analysis. Front Cardiovasc Med 8:778284. https://doi.org/10.3389/fcvm.2021.778284.
Razuk V, Chiarito M, Cao D, Nicolas J, Pivato CA, Camaj A et al (2022) SGLT-2 inhibitors and cardiovascular outcomes in patients with and without a history of heart failure: a systematic review and meta-analysis. Eur Hear Journal Cardiovasc Pharmacother 8:557–567. https://doi.org/10.1093/ehjcvp/pvac001
Singh AK, Singh R (2021) Cardiovascular Outcomes with SGLT-2 inhibitors in patients with heart failure with or without type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr Clin Res Rev 15:351–359. https://doi.org/10.1016/j.dsx.2021.01.006
Singh AK, Singh R, Misra A (2021) Do SGLT-2 inhibitors exhibit similar cardiovascular benefit in patients with heart failure with reduced or preserved ejection fraction? J Diabetes 13:596–600. https://doi.org/10.1111/1753-0407.13182
Staplin N, Roddick AJ, Emberson J, Reith C, Riding A, Wonnacott A et al (2021) Net effects of sodium-glucose co-transporter-2 inhibition in different patient groups: a meta-analysis of large placebo-controlled randomized trials. E Clinical Medicine 41. https://doi.org/10.1016/j.eclinm.2021.101163.
Tornyos D, Meuer M, Lukács R, El Alaoui El Abdallaoui O, Kupó P, Faludi R et al (2022) Cardiovascular outcomes in patients treated with sodium-glucose transport protein 2 inhibitors, a network meta-analysis of randomized trials. Front Cardiovasc Med 9. https://doi.org/10.3389/fcvm.2022.1041200
Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF et al (2022) SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet 400:757–767. https://doi.org/10.1016/S0140-6736(22)01429-5
Wang Y, Gao T, Meng C, Li S, Bi L, Geng Y et al (2022) Sodium-glucose co-transporter 2 inhibitors in heart failure with mildly reduced or preserved ejection fraction: an updated systematic review and meta-analysis. Eur J Med Res 27. https://doi.org/10.1186/s40001-022-00945-z
Yang D, Zhang Y, Yan J, Liu M, An F (2022) SGLT-2 inhibitors on prognosis and health-related quality of life in patients with heart failure and preserved ejection fraction: A systematic review and meta-analysis. Front Cardiovasc Med 9. https://doi.org/10.3389/fcvm.2022.942125.
Zhao L, Guo W, Huang W, Wang L, Huang S (2022) Benefit of sodium-glucose cotransporter-2 inhibitors on survival outcome is related to the type of heart failure: a meta-analysis. Diabetes Res Clin Pract 187. https://doi.org/10.1016/j.diabres.2022.109871
Zhao L-M, Ding L-L, Zhan Z-L, Qiu M (2021) Sotagliflozin reduces HF events in T2DM Regardless of baseline characteristics, including HF. CKD and LVEF Cardiovasc Drugs Ther 35:1077–1078. https://doi.org/10.1007/s10557-021-07203-0
Zheng C, Lin M, Chen Y, Xu H, Yan L, Dai H (2021) Effects of sodium-glucose cotransporter type 2 inhibitors on cardiovascular, renal, and safety outcomes in patients with cardiovascular disease: a meta-analysis of randomized controlled trials. Cardiovasc Diabetol 20. https://doi.org/10.1186/s12933-021-01272-z
Zhou H, Peng W, Li F, Wang Y, Wang B, Ding Y et al (2022) Effect of sodium-glucose cotransporter 2 inhibitors for heart failure with preserved ejection fraction: a systematic review and meta-analysis of randomized clinical trials. Front Cardiovasc Med 9. https://doi.org/10.3389/fcvm.2022.875327
Lou Y, Yang Q, Zhang W, Yu Y, Huang J (2022) Efficacy of sodium-glucose cotransporter 2 inhibitors in heart failure with a preserved ejection fraction: a meta-analysis of randomized controlled trials. Rev Cardiovasc Med 23:374. https://doi.org/10.31083/j.rcm2311374
Treewaree S, Kulthamrongsri N, Owattanapanich W, Krittayaphong R (2023) Is it time for class I recommendation for sodium-glucose cotransporter-2 inhibitors in heart failure with mildly reduced or preserved ejection fraction?: An updated systematic review and meta-analysis. Front Cardiovasc Med 10. https://doi.org/10.3389/FCVM.2023.1046194
Banerjee M, Pal R, Nair K, Mukhopadhyay S (2023) SGLT2 inhibitors and cardiovascular outcomes in heart failure with mildly reduced and preserved ejection fraction: a systematic review and meta-analysis. Indian Heart J. https://doi.org/10.1016/J.IHJ.2023.03.003
Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF et al (2022) Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 387:1089–1098. https://doi.org/10.1056/NEJMOA2206286/SUPPL_FILE/NEJMOA2206286_DATA-SHARING.PDF
Spertus JA, Jones PG, Sandhu AT, Arnold SV (2020) Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J Am Coll Cardiol 76:2379–2390. https://doi.org/10.1016/J.JACC.2020.09.542
Kennel PJ, Mancini DM, Schulze PC (2015) Skeletal muscle changes in chronic cardiac disease and failure. Compr Physiol 5:1947–1969. https://doi.org/10.1002/CPHY.C110003
Malhotra R, Bakken K, D’Elia E, Lewis GD (2016) Cardiopulmonary exercise testing in heart failure. JACC Hear Fail 4:607–616. https://doi.org/10.1016/J.JCHF.2016.03.022
Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S et al (2021) Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 77:243–255. https://doi.org/10.1016/J.JACC.2020.11.008
Author information
Authors and Affiliations
Contributions
Paschalis Karakasis: conceptualization, methodology, investigation, formal analysis, data curation, visualization, project administration, writing original draft, writing, review, and editing. Konstantinos Pamporis: methodology, writing original draft, writing, review, and editing. Panagiotis Stachteas: writing original draft, writing, review, and editing. Dimitrios Patoulias: writing original draft, writing, review, and editing. Konstantinos I. Bougioukas: methodology, writing original draft, writing, review, and editing. Nikolaos Fragakis: conceptualization, methodology, validation, writing, review, editing, and supervision.
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Protocol
The study protocol was prospectively registered in OSF (https://osf.io/p6a9w/).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karakasis, P., Pamporis, K., Stachteas, P. et al. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors in heart failure with mildly reduced or preserved ejection fraction: an overview of 36 systematic reviews. Heart Fail Rev 28, 1033–1051 (2023). https://doi.org/10.1007/s10741-023-10324-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-023-10324-3