Abstract
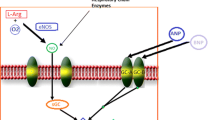
The nitric oxide (NO)–guanylate cyclase (GC)–cyclic guanosine monophosphate (cGMP) pathway plays an important role in cardiovascular, pulmonary and renal function. Phosphodiesterase-5 inhibitors (PDE-5i) inhibit cGMP degradation, whereas both soluble guanylate cyclase (sGC) stimulators and sGC activators directly increase sGC. PDE-5i (e.g. sildenafil, tadalafil) and sGC stimulators (e.g. riociguat, vericiguat) have been extensively used in pulmonary artery hypertension (PAH) and heart failure (HF). PDE-5i have also been used in end-stage HF before and after left ventricular (LV) assist device (LVAD) implantation. Augmentation of NO-GC-cGMP signalling with PDE-5i causes selective pulmonary vasodilation, which is highly effective in PAH but may have controversial, potentially adverse effects in HF, including pre-LVAD implant due to device unmasking of PDE-5i-induced RV dysfunction. In contrast, retrospective analyses have demonstrated that PDE-5i have beneficial effects when initiated post LVAD implant due to the improved haemodynamics of the supported LV and the pleiotropic actions of these compounds. sGC stimulators, in turn, are effective both in PAH and in HF due to their balanced pulmonary and systemic vasodilation, and as such they are preferable to PDE-5i if the use of a pulmonary vasodilator is needed in HF patients, including those listed for LVAD implantation. Regarding the effectiveness of PDE-5i and sGC stimulators when initiated post LVAD implant, these two groups of compounds should be tested in a randomized control trial.






Similar content being viewed by others
References
Hofmann F (2020) The cGMP system: components and function. Biol Chem 401(4):447–469
Frantz RP (2021) Replace and the role of riociguat in pulmonary arterial hypertension therapy. Lancet Respir Med 9(6):546–547
Markham A, Duggan S (2021) Vericiguat: first approval. Drugs 81(6):721–726
Xanthopoulos A, Tryposkiadis K, Triposkiadis F, Fukamachi K, Soltesz EG, Young JB, Wolski K, Blackstone EH, Starling RC (2020) Postimplant phosphodiesterase type 5 inhibitors use is associated with lower rates of thrombotic events after left ventricular assist device implantation. J Am Heart Assoc 9(14):e015897
Xanthopoulos A, Wolski K, Wang Q, Blackstone EH, Randhawa VK, Soltesz EG, Young JB, Nissen SE, Estep JD, Triposkiadis F et al (2022) Postimplant phosphodiesterase-5 inhibitor use in centrifugal flow left ventricular assist devices. JACC Heart Fail 10(2):89–100
Lai YC, Potoka KC, Champion HC, Mora AL, Gladwin MT (2014) Pulmonary arterial hypertension: the clinical syndrome. Circ Res 115(1):115–130
Galie N, Manes A, Dardi F, Palazzini M (2018) Aiming at the appropriate target for the treatment of pulmonary hypertension due to left heart disease. Eur Heart J 39(15):1265–1268
Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R (2019) Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 53(1)
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M et al (2015) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (ESC) and the european respiratory society (ERS): Endorsed by: association for european paediatric and congenital cardiology (AEPC), International society for heart and lung transplantation (ISHLT). Eur Respir J 46(4):903–975
Macera F, Vachiery JL (2021) Management of pulmonary hypertension in left heart disease. Methodist Debakey Cardiovasc J 17(2):115–123
Alamri AK, Ma CL, Ryan JJ (2022) Left heart disease-related pulmonary hypertension. Cardiol Clin 40(1):69–76
Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM, Redfield MM (2018) Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation 137(17):1796–1810
Lee F, Mielniczuk LM (2021) Pulmonary hypertension due to left heart disease-a practical approach to diagnosis and management. Can J Cardiol 37(4):572–584
Fernandez AI, Yotti R, Gonzalez-Mansilla A, Mombiela T, Gutierrez-Ibanes E, Perez Del Villar C, Navas-Tejedor P, Chazo C, Martinez-Legazpi P, Fernandez-Aviles F et al (2019) The biological bases of group 2 pulmonary hypertension. Int J Mol Sci 20(23)
Al-Omary MS, Sugito S, Boyle AJ, Sverdlov AL, Collins NJ (2020) Pulmonary hypertension due to left heart disease: diagnosis, pathophysiology, and therapy. Hypertension 75(6):1397–1408
Vachiery JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, Coghlan G, Chazova I, De Marco T (2019) Pulmonary hypertension due to left heart disease. Eur Respir J 53(1)
Duran A, Mandras S (2021) Pulmonary hypertension in heart failure. Curr Opin Cardiol 36(2):205–210
Opitz CF, Hoeper MM, Gibbs JS, Kaemmerer H, Pepke-Zaba J, Coghlan JG, Scelsi L, D’Alto M, Olsson KM, Ulrich S et al (2016) Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 68(4):368–378
Selim AM, Wadhwani L, Burdorf A, Raichlin E, Lowes B, Zolty R (2019) Left ventricular assist devices in pulmonary hypertension group 2 with significantly elevated pulmonary vascular resistance: a bridge to cure. Heart Lung Circ 28(6):946–952
Arrigo M, Huber LC, Winnik S, Mikulicic F, Guidetti F, Frank M, Flammer AJ, Ruschitzka F (2019) Right ventricular failure: pathophysiology, diagnosis and treatment. Card Fail Rev 5(3):140–146
Haddad F, Doyle R, Murphy DJ, Hunt SA (2008) Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 117(13):1717–1731
Haddad F, Hunt SA, Rosenthal DN, Murphy DJ (2008) Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 117(11):1436–1448
Naeije R, Manes A (2014) The right ventricle in pulmonary arterial hypertension. Eur Respir Rev 23(134):476–487
Ryan JJ, Archer SL (2014) The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 115(1):176–188
Naeije R, Richter MJ, Rubin LJ (2021) The physiologic basis of pulmonary arterial hypertension. Eur Respir J
Querejeta Roca G, Campbell P, Claggett B, Solomon SD, Shah AM (2015) Right atrial function in pulmonary arterial hypertension. Circ Cardiovasc Imaging 8(11):e003521; discussion e003521
Marra AM, Sherman AE, Salzano A, Guazzi M, Saggar R, Squire IB, Cittadini A, Channick RN, Bossone E (2022) Right side of the heart pulmonary circulation unit involvement in left-sided heart failure: Diagnostic, prognostic, and therapeutic implications. Chest 161(2):535–551
Pagani FD (2020) Right heart failure after left ventricular assist device placement: medical and surgical management considerations. Cardiol Clin 38(2):227–238
Hardziyenka M, Campian ME, Reesink HJ, Surie S, Bouma BJ, Groenink M, Klemens CA, Beekman L, Remme CA, Bresser P et al (2011) Right ventricular failure following chronic pressure overload is associated with reduction in left ventricular mass: evidence for atrophic remodeling. J Am Coll Cardiol 57(8):921–928
Kishiki K, Singh A, Narang A, Gomberg-Maitland M, Goyal N, Maffessanti F, Besser SA, Mor-Avi V, Lang RM, Addetia K (2019) Impact of severe pulmonary arterial hypertension on the left heart and prognostic implications. J Am Soc Echocardiogr 32(9):1128–1137
Hirashiki A, Adachi S, Nakano Y, Kamimura Y, Ogo T, Nakanishi N, Morisaki T, Morisaki H, Shimizu A, Toba K et al (2017) Left main coronary artery compression by a dilated main pulmonary artery and left coronary sinus of Valsalva aneurysm in a patient with heritable pulmonary arterial hypertension and FLNA mutation. Pulm Circ 7(3):734–740
Katz AM, Rolett EL (2016) Heart failure: when form fails to follow function. Eur Heart J 37(5):449–454
Triposkiadis F, Pieske B, Butler J, Parissis J, Giamouzis G, Skoularigis J, Brutsaert D, Boudoulas H (2016) Global left atrial failure in heart failure. Eur J Heart Fail 18(11):1307–1320
Khan MS, Memon MM, Murad MH, Vaduganathan M, Greene SJ, Hall M, Triposkiadis F, Lam CSP, Shah AM, Butler J et al (2020) Left atrial function in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail 22(3):472–485
Del Rio JM, Grecu L, Nicoara A (2019) Right ventricular function in left heart disease. Semin Cardiothorac Vasc Anesth 23(1):88–107
Wanner PM, Filipovic M (2020) The right ventricle-you may forget it, but it will not forget you. J Clin Med 9(2)
Janicki JS (1990) Influence of the pericardium and ventricular interdependence on left ventricular diastolic and systolic function in patients with heart failure. Circulation 81(2 Suppl):III15–20
Borlaug BA, Reddy YNV (2019) The role of the pericardium in heart failure: implications for pathophysiology and treatment. JACC Heart Fail 7(7):574–585
Hoffman D, Sisto D, Frater RW, Nikolic SD (1994) Left-to-right ventricular interaction with a noncontracting right ventricle. J Thorac Cardiovasc Surg 107(6):1496–1502
Bernardo RJ, Haddad F, Couture EJ, Hansmann G, de Jesus Perez VA, Denault AY, de Man FS, Amsallem M (2020) Mechanics of right ventricular dysfunction in pulmonary arterial hypertension and heart failure with preserved ejection fraction. Cardiovasc Diagn Ther 10(5):1580–1603
Sack KL, Dabiri Y, Franz T, Solomon SD, Burkhoff D, Guccione JM (2018) Investigating the role of interventricular interdependence in development of right heart dysfunction during LVAD support: a patient-specific methods-based approach. Front Physiol 9:520
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837, 837a–837d
Preedy MEJ (2020) Cardiac cyclic nucleotide phosphodiesterases: roles and therapeutic potential in heart failure. Cardiovasc Drugs Ther 34(3):401–417
Kraehling JR, Sessa WC (2017) Contemporary approaches to modulating the nitric oxide-cGMP pathway in cardiovascular disease. Circ Res 120(7):1174–1182
Farah C, Michel LYM, Balligand JL (2018) Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol 15(5):292–316
Munzel T, Feil R, Mulsch A, Lohmann SM, Hofmann F, Walter U (2003) Physiology and pathophysiology of vascular signaling controlled by guanosine 3’,5’-cyclic monophosphate-dependent protein kinase [corrected]. Circulation 108(18):2172–2183
Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F (2003) Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res 93(10):907–916
Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G (2006) Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res 99(8):816–828
Azevedo MF, Faucz FR, Bimpaki E, Horvath A, Levy I, de Alexandre RB, Ahmad F, Manganiello V, Stratakis CA (2014) Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr Rev 35(2):195–233
Chester AH, Yacoub MH, Moncada S (2017) Nitric oxide and pulmonary arterial hypertension. Glob Cardiol Sci Pract 2017(2):14
Klinger JR, Kadowitz PJ (2017) The nitric oxide pathway in pulmonary vascular disease. Am J Cardiol 120(8S):S71–S79
Giaid A, Saleh D (1995) Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 333(4):214–221
Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, Yacoub MH, Polak JM (1998) High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Pathol 185(3):313–318
Stasch JP, Pacher P, Evgenov OV (2011) Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123(20):2263–2273
Kuhn M (2016) Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev 96(2):751–804
Blanton RM (2020) cGMP signaling and modulation in heart failure. J Cardiovasc Pharmacol 75(5):385–398
Triposkiadis F, Xanthopoulos A, Butler J (2020) From Paradigm to Paragon further evidence supporting continuous heart failure spectrum. Eur J Heart Fail 22(9):1536–1539
Kong Q, Blanton RM (2013) Protein kinase G I and heart failure: shifting focus from vascular unloading to direct myocardial antiremodeling effects. Circ Heart Fail 6(6):1268–1283
Sansbury BE, Hill BG (2014) Regulation of obesity and insulin resistance by nitric oxide. Free Radic Biol Med 73:383–399
Paolocci N, Biondi R, Bettini M, Lee CI, Berlowitz CO, Rossi R, Xia Y, Ambrosio G, L’Abbate A, Kass DA et al (2001) Oxygen radical-mediated reduction in basal and agonist-evoked NO release in isolated rat heart. J Mol Cell Cardiol 33(4):671–679
Karbach S, Wenzel P, Waisman A, Munzel T, Daiber A (2014) eNOS uncoupling in cardiovascular diseases–the role of oxidative stress and inflammation. Curr Pharm Des 20(22):3579–3594
Ignarro LJ, Napoli C, Loscalzo J (2002) Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: an overview. Circ Res 90(1):21–28
Follmann M, Griebenow N, Hahn MG, Hartung I, Mais FJ, Mittendorf J, Schafer M, Schirok H, Stasch JP, Stoll F et al (2013) The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed Engl 52(36):9442–9462
Keravis T, Lugnier C (2012) Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol 165(5):1288–1305
Hutchings DC, Anderson SG, Caldwell JL, Trafford AW (2018) Phosphodiesterase-5 inhibitors and the heart: compound cardioprotection? Heart 104(15):1244–1250
Sandner P, Follmann M, Becker-Pelster E, Hahn MG, Meier C, Freitas C, Roessig L, Stasch JP (2021) Soluble GC stimulators and activators: past, present and future. Br J Pharmacol
Cordwin DJ, Berei TJ, Pogue KT (2021) The role of sGC stimulators and activators in heart failure with reduced ejection fraction. J Cardiovasc Pharmacol Ther 26(6):593–600
Sandner P, Zimmer DP, Milne GT, Follmann M, Hobbs A, Stasch JP (2021) Soluble guanylate cyclase stimulators and activators. Handb Exp Pharmacol 264:355–394
Hassoun PM (2021) Pulmonary arterial hypertension. N Engl J Med 385(25):2361–2376
Mayeux JD, Pan IZ, Dechand J, Jacobs JA, Jones TL, McKellar SH, Beck E, Hatton ND, Ryan JJ (2021) Management of pulmonary arterial hypertension. Curr Cardiovasc Risk Rep 15(1):2
Watanabe H (2018) Treatment selection in pulmonary arterial hypertension: phosphodiesterase type 5 inhibitors versus soluble guanylate cyclase stimulator. Eur Cardiol 13(1):35–37
Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD et al (2007) Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 116(14):1555–1562
Belyavskiy E, Ovchinnikov A, Potekhina A, Ageev F, Edelmann F (2020) Phosphodiesterase 5 inhibitor sildenafil in patients with heart failure with preserved ejection fraction and combined pre- and postcapillary pulmonary hypertension: a randomized open-label pilot study. BMC Cardiovasc Disord 20(1):408
Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL et al (2013) Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309(12):1268–1277
Bermejo J, Yotti R, Garcia-Orta R, Sanchez-Fernandez PL, Castano M, Segovia-Cubero J, Escribano-Subias P, San Roman JA, Borras X, Alonso-Gomez A et al (2018) Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur Heart J 39(15):1255–1264
Ravichandran AK, LaRue SJ, Novak E, Joseph SA, Schilling JD (2018) Sildenafil in left ventricular assist device is safe and well-tolerated. ASAIO J 64(2):280–281
Gulati G, Grandin EW, Kennedy K, Cabezas F, DeNofrio DD, Kociol R, Rame JE, Pagani FD, Kirklin JK, Kormos RL et al (2019) Preimplant phosphodiesterase-5 inhibitor use is associated with higher rates of severe early right heart failure after left ventricular assist device implantation. Circ Heart Fail 12(6):e005537
Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis E, Mitrovic V, Oudiz RJ, Boateng F, Scalise AV, Roessig L et al (2013) Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation 128(5):502–511
Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E et al (2015) Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the socrates-reduced randomized trial. JAMA 314(21):2251–2262
Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G et al (2020) Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 382(20):1883–1893
Lam CSP, Mulder H, Lopatin Y, Vazquez-Tanus JB, Siu D, Ezekowitz J, Pieske B, O'Connor CM, Roessig L, Patel MJ et al (2021) Blood pressure and safety events with vericiguat in the victoria trial. J Am Heart Assoc 10(22):e021094
Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S (2002) Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation 105(20):2398–2403
Monzo L, Reichenbach A, Al-Hiti H, Borlaug BA, Havlenova T, Solar N, Tupy M, Ters J, Kautzner J, Melenovsky V (2021) Acute unloading effects of sildenafil enhance right ventricular-pulmonary artery coupling in heart failure. J Card Fail 27(2):224–232
Lincoln TM, Hall CL, Park CR, Corbin JD (1976) Guanosine 3’:5’-cyclic monophosphate binding proteins in rat tissues. Proc Natl Acad Sci U S A 73(8):2559–2563
Corbin JD, Beasley A, Blount MA, Francis SH (2005) High lung PDE5: a strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun 334(3):930–938
Wharton J, Strange JW, Moller GM, Growcott EJ, Ren X, Franklyn AP, Phillips SC, Wilkins MR (2005) Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med 172(1):105–113
Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, van Veldhuisen DJ, Voors AA (2015) Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J 36(38):2565–2573
Reddy YNV, El-Sabbagh A, Nishimura RA (2018) Comparing pulmonary arterial wedge pressure and left ventricular end diastolic pressure for assessment of left-sided filling pressures. JAMA Cardiol 3(6):453–454
Hemnes AR, Opotowsky AR, Assad TR, Xu M, Doss LN, Farber-Eger E, Wells QS, Brittain EL (2018) Features associated with discordance between pulmonary arterial wedge pressure and left ventricular end diastolic pressure in clinical practice: implications for pulmonary hypertension classification. Chest 154(5):1099–1107
Schranz D, Rupp S, Muller M, Schmidt D, Bauer A, Valeske K, Michel-Behnke I, Jux C, Apitz C, Thul J et al (2013) Pulmonary artery banding in infants and young children with left ventricular dilated cardiomyopathy: a novel therapeutic strategy before heart transplantation. J Heart Lung Transplant 32(5):475–481
Schranz D, Akintuerk H, Bailey L (2018) Pulmonary artery banding for functional regeneration of end-stage dilated cardiomyopathy in young children: World Network Report. Circulation 137(13):1410–1412
Di Candia A, Castaldi B, Bordin G, Cerutti A, Reffo E, Biffanti R, Di Salvo G, Vida VL, Padalino MA (2020) Pulmonary artery banding for ventricular rehabilitation in infants with dilated cardiomyopathy: early results in a single-center experience. Front Pediatr 8:347
Schranz D, Recla S, Malcic I, Kerst G, Mini N, Akintuerk H (2019) Pulmonary artery banding in dilative cardiomyopathy of young children: review and protocol based on the current knowledge. Transl Pediatr 8(2):151–160
Neragi-Miandoab S, Goldstein D, Bello R, Michler R, D’Alessandro D (2012) Right ventricular dysfunction following continuous flow left ventricular assist device placement in 51 patients: predicators and outcomes. J Cardiothorac Surg 7:60
Magoon R, Jose J, Kohli JK, Kashav R (2020) Altered RV mechanics post-LVAD insertion: a physiological perspective! Braz J Cardiovasc Surg 35(3):407–408
El-Sayed MI, Amin HA (2015) Mechanism of endothelial cyto-protective and thrombo-resistance effects of sildenafil, vardenafil and tadalafil in male rabbit. Arch Med Sci 11(1):190–198
Naesheim T, How OJ, Myrmel T (2021) Hemodynamic effects of a soluble guanylate cyclase stimulator, riociguat, and an activator, cinaciguat, during NO-modulation in healthy pigs. J Cardiovasc Pharmacol Ther 26(1):75–87
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Triposkiadis, F., Xanthopoulos, A., Skoularigis, J. et al. Therapeutic augmentation of NO-sGC-cGMP signalling: lessons learned from pulmonary arterial hypertension and heart failure. Heart Fail Rev 27, 1991–2003 (2022). https://doi.org/10.1007/s10741-022-10239-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-022-10239-5