Abstract
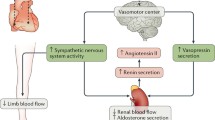
Neurohormones and inflammatory mediators have effects in both the heart and the peripheral vasculature. In patients with heart failure (HF), neurohormonal activation and increased levels of inflammatory mediators promote ventricular remodeling and development of HF, as well as vascular dysfunction and arterial stiffness. These processes may lead to a vicious cycle, whereby arterial stiffness perpetuates further ventricular remodeling leading to exacerbation of symptoms. Although significant advances have been made in the treatment of HF, currently available treatment strategies slow, but do not halt, this cycle. The current treatment for HF patients involves the inhibition of neurohormonal activation, which can reduce morbidity and mortality related to this condition. Beyond benefits associated with neurohormonal blockade, other strategies have focused on inhibition of inflammatory pathways implicated in the pathogenesis of HF. Unfortunately, attempts to target inflammation have not yet been successful to improve prognosis of HF. Further work is required to interrupt key maladaptive mechanisms involved in disease progression.

Similar content being viewed by others
References
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62(16):e147–e239. https://doi.org/10.1016/j.jacc.2013.05.019
Krum H, Abraham WT (2009) Heart failure. Lancet 373(9667):941–955. https://doi.org/10.1016/S0140-6736(09)60236-1
Jessup M, Brozena S (2003) Heart failure. N Engl J Med 348(20):2007–2018. https://doi.org/10.1056/NEJMra021498
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol 35(3):569–582
Mann DL, Bristow MR (2005) Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 111(21):2837–2849. https://doi.org/10.1161/CIRCULATIONAHA.104.500546
Schrier RW, Abraham WT (1999) Hormones and hemodynamics in heart failure. N Engl J Med 341(8):577–585. https://doi.org/10.1056/NEJM199908193410806
Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB (1982) Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307(4):205–211. https://doi.org/10.1056/NEJM198207223070401
Florea VG, Cohn JN (2014) The autonomic nervous system and heart failure. Circ Res 114(11):1815–1826. https://doi.org/10.1161/circresaha.114.302589
Lamba S, Abraham WT (2000) Alterations in adrenergic receptor signaling in heart failure. Heart Fail Rev 5(1):7–16. https://doi.org/10.1023/A:1009885822076
Communal C, Singh K, Pimentel DR, Colucci WS (1998) Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation 98(13):1329–1334
Mann DL, Kent RL, Parsons B, Cooper G (1992) Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 85(2):790–804
Simpson P (1983)Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest 72(2):732–738. https://doi.org/10.1172/JCI111023
Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T (1984) Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311(13):819–823. https://doi.org/10.1056/NEJM198409273111303
Lechat P et al (1999) The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 353(9146):9–13
Maxwell AP, Ong HY, Nicholls DP (2002) Influence of progressive renal dysfunction in chronic heart failure. Eur J Heart Fail 4(2):125–130
Weber KT (2001) Aldosterone in congestive heart failure. N Engl J Med 345(23):1689–1697. https://doi.org/10.1056/NEJMra000050
Sequeira-Lopez ML, Weatherford ET, Borges GR, Monteagudo MC, Pentz ES, Harfe BD, Carretero O, Sigmund CD, Gomez RA (2010) The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol 21(3):460–467. https://doi.org/10.1681/ASN.2009090964
Masson S, Solomon S, Angelici L, Latini R, Anand IS, Prescott M, Maggioni AP, Tognoni G, Cohn JN, Val-Heft I (2010) Elevated plasma renin activity predicts adverse outcome in chronic heart failure, independently of pharmacologic therapy: data from the valsartan heart failure trial (Val-HeFT). J Card Fail 16(12):964–970. https://doi.org/10.1016/j.cardfail.2010.06.417
Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L (1990) Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS trial study group. Circulation 82(5):1730–1736
Reid IA (1992) Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Phys 262(6 Pt 1):E763–E778
Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump C, Ferrario C, Sowers JR (2007) Angiotensin II-mediated oxidative stress promotes myocardial tissue remodeling in the transgenic (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab 293(1):E355–E363. https://doi.org/10.1152/ajpendo.00632.2006
Sadoshima J, Izumo S (1993) Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res 73(3):413–423
Kawano H, Do YS, Kawano Y, Starnes V, Barr M, Law RE, Hsueh WA (2000) Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation 101(10):1130–1137
Booz GW, Baker KM (1995) Molecular signalling mechanisms controlling growth and function of cardiac fibroblasts. Cardiovasc Res 30(4):537–543
Harada K, Komuro I, Shiojima I, Hayashi D, Kudoh S, Mizuno T, Kijima K, Matsubara H, Sugaya T, Murakami K, Yazaki Y (1998) Pressure overload induces cardiac hypertrophy in angiotensin II type 1A receptor knockout mice. Circulation 97(19):1952–1959
Ruzicka M, Leenen FH (1995) Relevance of blockade of cardiac and circulatory angiotensin-converting enzyme for the prevention of volume overload-induced cardiac hypertrophy. Circulation 91(1):16–19
Sadoshima J, Xu Y, Slayter HS, Izumo S (1993) Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 75(5):977–984
Reudelhuber TL, Bernstein KE, Delafontaine P (2007) Is angiotensin II a direct mediator of left ventricular hypertrophy? Time for another look. Hypertension 49(6):1196–1201. https://doi.org/10.1161/hypertensionaha.106.075085
Mizuno Y, Yoshimura M, Yasue H, Sakamoto T, Ogawa H, Kugiyama K, Harada E, Nakayama M, Nakamura S, Ito T, Shimasaki Y, Saito Y, Nakao K (2001) Aldosterone production is activated in failing ventricle in humans. Circulation 103(1):72–77
Hatakeyama H, Miyamori I, Fujita T, Takeda Y, Takeda R, Yamamoto H (1994) Vascular aldosterone. Biosynthesis and a link to angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem 269(39):24316–24320
Schiffrin EL (2006) Effects of aldosterone on the vasculature. Hypertension 47(3):312–318. https://doi.org/10.1161/01.HYP.0000201443.63240.a7
Yoshida Y, Morimoto T, Takaya T, Kawamura T, Sunagawa Y, Wada H, Fujita M, Shimatsu A, Kita T, Hasegawa K (2010) Aldosterone signaling associates with p300/GATA4 transcriptional pathway during the hypertrophic response of cardiomyocytes. Circ J 74(1):156–162
Okoshi MP, Yan X, Okoshi K, Nakayama M, Schuldt AJ, O’Connell TD, Simpson PC, Lorell BH (2004) Aldosterone directly stimulates cardiac myocyte hypertrophy. J Card Fail 10(6):511–518
Brilla CG, Zhou G, Matsubara L, Weber KT (1994) Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol 26(7):809–820. https://doi.org/10.1006/jmcc.1994.1098
Brilla CG, Matsubara LS, Weber KT (1993)Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol 25(5):563–575. https://doi.org/10.1006/jmcc.1993.1066
Glasser SP (2000) On arterial physiology, pathophysiology of vascular compliance, and cardiovascular disease. Heart Dis (Hagerstown, Md) 2(5):375–379
Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C (2002) Clinical applications of arterial stiffness, task force III: recommendations for user procedures. Am J Hypertens 15(5):445–452
Zieman SJ, Melenovsky V, Kass DA (2005) Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25(5):932–943. https://doi.org/10.1161/01.ATV.0000160548.78317.29
Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM (1991)Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation 84(4):1589–1596
Drexler H, Hayoz D, Munzel T, Just H, Zelis R, Brunner HR (1993) Endothelial function in congestive heart failure. Am Heart J 126(3 Pt 2):761–764
Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H (2005) Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J 26(1):65–69. https://doi.org/10.1093/eurheartj/ehi001
McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, Cockcroft JR, Wilkinson IB (2006) Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 48(4):602–608. https://doi.org/10.1161/01.HYP.0000239206.64270.5f
Fitch RM, Vergona R, Sullivan ME, Wang YX (2001) Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc Res 51(2):351–358
Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR (2002) Nitric oxide regulates local arterial distensibility in vivo. Circulation 105(2):213–217
Verma S, Buchanan MR, Anderson TJ (2003) Endothelial function testing as a biomarker of vascular disease. Circulation 108(17):2054–2059. https://doi.org/10.1161/01.CIR.0000089191.72957.ED
Ramsey MW, Goodfellow J, Jones CJ, Luddington LA, Lewis MJ, Henderson AH (1995) Endothelial control of arterial distensibility is impaired in chronic heart failure. Circulation 92(11):3212–3219
Nakamura M, Funakoshi T, Yoshida H, Arakawa N, Suzuki T, Hiramori K (1992)Endothelium-dependent vasodilation is augmented by angiotensin converting enzyme inhibitors in healthy volunteers. J Cardiovasc Pharmacol 20(6):949–954
Lage SG, Kopel L, Monachini MC, Medeiros CJ, Pileggi F, Polak JF, Creager MA (1994) Carotid arterial compliance in patients with congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol 74(7):691–695
Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A (2005) Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation 111(3):310–314. https://doi.org/10.1161/01.CIR.0000153349.77489.CF
Yambe M, Tomiyama H, Hirayama Y, Gulniza Z, Takata Y, Koji Y, Motobe K, Yamashina A (2004) Arterial stiffening as a possible risk factor for both atherosclerosis and diastolic heart failure. Hypertens Res 27(9):625–631
Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A, Health ABCS (2005) Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111(25):3384–3390. https://doi.org/10.1161/CIRCULATIONAHA.104.483628
Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J (2006) Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 113(5):664–670. https://doi.org/10.1161/CIRCULATIONAHA.105.579342
Bonapace S, Rossi A, Cicoira M, Franceschini L, Golia G, Zanolla L, Marino P, Zardini P (2003) Aortic distensibility independently affects exercise tolerance in patients with dilated cardiomyopathy. Circulation 107(12):1603–1608. https://doi.org/10.1161/01.CIR.0000051458.39176.43
Desai AS, Mitchell GF, Fang JC, Creager MA (2009) Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail 15(8):658–664. https://doi.org/10.1016/j.cardfail.2009.03.006
Nakamura M, Sugawara S, Arakawa N, Nagano M, Shizuka T, Shimoda Y, Sakai T, Hiramori K (2004) Reduced vascular compliance is associated with impaired endothelium-dependent dilatation in the brachial artery of patients with congestive heart failure. J Card Fail 10(1):36–42
Arnold JM, Marchiori GE, Imrie JR, Burton GL, Pflugfelder PW, Kostuk WJ (1991) Large artery function in patients with chronic heart failure. Studies of brachial artery diameter and hemodynamics. Circulation 84(6):2418–2425
Giannattasio C, Failla M, Stella ML, Mangoni AA, Turrini D, Carugo S, Pozzi M, Grassi G, Mancia G (1995)Angiotensin-converting enzyme inhibition and radial artery compliance in patients with congestive heart failure. Hypertension 26(3):491–496
Giannattasio C, Achilli F, Failla M, Capra A, Vincenzi A, Valagussa F, Mancia G (2002) Radial, carotid and aortic distensibility in congestive heart failure: effects of high-dose angiotensin-converting enzyme inhibitor or low-dose association with angiotensin type 1 receptor blockade. J Am Coll Cardiol 39(8):1275–1282
Patrianakos AP, Parthenakis FI, Karakitsos D, Nyktari E, Vardas PE (2009) Proximal aortic stiffness is related to left ventricular function and exercise capacity in patients with dilated cardiomyopathy. Eur J Echocardiogr 10(3):425–432. https://doi.org/10.1093/ejechocard/jen304
Rerkpattanapipat P, Hundley WG, Link KM, Brubaker PH, Hamilton CA, Darty SN, Morgan TM, Kitzman DW (2002) Relation of aortic distensibility determined by magnetic resonance imaging in patients > or =60 years of age to systolic heart failure and exercise capacity. Am J Cardiol 90(11):1221–1225
Ooi H, Chung W, Biolo A (2008) Arterial stiffness and vascular load in heart failure. Congest Heart Fail (Greenwich, Conn) 14(1):31–36
Safar ME, Lacolley P (2007) Disturbance of macro- and microcirculation: relations with pulse pressure and cardiac organ damage. Am J Physiol Heart Circ Physiol 293(1):H1–H7. https://doi.org/10.1152/ajpheart.00063.2007
Saito M, Okayama H, Nishimura K, Ogimoto A, Ohtsuka T, Inoue K, Hiasa G, Sumimoto T, Higaki J (2008) Possible link between large artery stiffness and coronary flow velocity reserve. Heart 94(6):e20. https://doi.org/10.1136/hrt.2007.126128
Babalis D, Levy BI, Azancot I, Masquet C, Beaufils P (1984) Ventricular function and arterial compliance in patients with congestive cardiomyopathy. Int J Cardiol 5(3):361–364
Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P (2005) Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol 46(2):291–298. https://doi.org/10.1016/j.jacc.2004.10.081
Maruyama Y, Nishioka O, Nozaki E, Kinoshita H, Kyono H, Koiwa Y, Takishima T (1993) Effects of arterial distensibility on left ventricular ejection in the depressed contractile state. Cardiovasc Res 27(2):182–187
Grassi G, Giannattasio C, Failla M, Pesenti A, Peretti G, Marinoni E, Fraschini N, Vailati S, Mancia G (1995) Sympathetic modulation of radial artery compliance in congestive heart failure. Hypertension 26(2):348–354
Wilson RA, Di Mario C, Krams R, Soei LK, Wenguang L, Laird AC, The SH, Gussenhoven E, Verdouw P, Roelandt JR (1995) In vivo measurement of regional large artery compliance by intravascular ultrasound under pentobarbital anesthesia. Angiology 46(6):481–488
Cadnapaphornchai MA, Gurevich AK, Weinberger HD, Schrier RW (2001) Pathophysiology of sodium and water retention in heart failure. Cardiology 96(3–4):122–131
Patrianakos AP, Parthenakis FI, Nyktari E, Malliaraki N, Karakitsos DN, Vardas PE (2009) Central aortic stiffness in patients with nonischemic dilated cardiomyopathy: relationship with neurohumoral activation. J Card Fail 15(8):665–672. https://doi.org/10.1016/j.cardfail.2009.03.007
Kranzhofer R, Schmidt J, Pfeiffer CA, Hagl S, Libby P, Kubler W (1999) Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19(7):1623–1629
Tummala PE, Chen XL, Sundell CL, Laursen JB, Hammes CP, Alexander RW, Harrison DG, Medford RM (1999) Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: a potential link between the renin-angiotensin system and atherosclerosis. Circulation 100(11):1223–1229
Guo F, Chen XL, Wang F, Liang X, Sun YX, Wang YJ (2011) Role of angiotensin II type 1 receptor in angiotensin II-induced cytokine production in macrophages. Interferon Cytokine Res 31(4):351–361. https://doi.org/10.1089/jir.2010.0073
Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM (1998) Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res 83(9):952–959
Caprio M, Newfell BG, la Sala A, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ (2008) Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res 102(11):1359–1367. https://doi.org/10.1161/CIRCRESAHA.108.174235
Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, Brown NJ (2006) Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension 48(6):1050–1057. https://doi.org/10.1161/01.HYP.0000248135.97380.76
Arenas IA, Xu Y, Lopez-Jaramillo P, Davidge ST (2004) Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-alpha. Am J Physiol Cell Physiol 286(4):C779–C784. https://doi.org/10.1152/ajpcell.00398.2003
Li M, Liu J, Han C, Wang B, Pang X, Mao J (2011) Angiotensin II induces the expression of c-reactive protein via MAPK-dependent signal pathway in U937 macrophages. Cell Physiol Biochem 27(1):63–70. https://doi.org/10.1159/000325206
Peng N, Liu JT, Gao DF, Lin R, Li R (2007) Angiotensin II-induced C-reactive protein generation: inflammatory role of vascular smooth muscle cells in atherosclerosis. Atherosclerosis 193(2):292–298. https://doi.org/10.1016/j.atherosclerosis.2006.09.007
Touyz RM, Schiffrin EL (1999) Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension 34(4 Pt 2):976–982
Touyz RM, Cruzado M, Tabet F, Yao G, Salomon S, Schiffrin EL (2003)Redox-dependent MAP kinase signaling by Ang II in vascular smooth muscle cells: role of receptor tyrosine kinase transactivation. Can J Physiol Pharmacol 81(2):159–167. https://doi.org/10.1139/y02-164
Loot AE, Schreiber JG, Fisslthaler B, Fleming I (2009) Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J Exp Med 206(13):2889–2896. https://doi.org/10.1084/jem.20090449
Szabo C, Pacher P, Zsengeller Z, Vaslin A, Komjati K, Benko R, Chen M, Mabley JG, Kollai M (2004) Angiotensin II-mediated endothelial dysfunction: role of poly(ADP-ribose) polymerase activation. Mol Med (Cambridge, Mass) 10(1–6):28–35
Kato H, Suzuki H, Tajima S, Ogata Y, Tominaga T, Sato A, Saruta T (1991) Angiotensin II stimulates collagen synthesis in cultured vascular smooth muscle cells. J Hypertens 9(1):17-22
Daemen MJ, Lombardi DM, Bosman FT, Schwartz SM (1991) Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res 68(2):450–456
Rehman A, Rahman AR, Rasool AH (2002) Effect of angiotensin II on pulse wave velocity in humans is mediated through angiotensin II type 1 (AT(1)) receptors. J Hum Hypertens 16(4):261–266. https://doi.org/10.1038/sj.jhh.1001372
Duprez DA, De Buyzere ML, Rietzschel ER, Taes Y, Clement DL, Morgan D, Cohn JN (1998) Inverse relationship between aldosterone and large artery compliance in chronically treated heart failure patients. Eur Heart J 19(9):1371–1376
Callera GE, Touyz RM, Tostes RC, Yogi A, He Y, Malkinson S, Schiffrin EL (2005) Aldosterone activates vascular p38MAP kinase and NADPH oxidase via c-Src. Hypertension 45(4):773–779. https://doi.org/10.1161/01.HYP.0000154365.30593.d3
Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, Hirata Y, Naruse M (2006) Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension 48(1):165–171. https://doi.org/10.1161/01.HYP.0000226054.53527.bb
Hashikabe Y, Suzuki K, Jojima T, Uchida K, Hattori Y (2006) Aldosterone impairs vascular endothelial cell function. J Cardiovasc Pharmacol 47(4):609–613. https://doi.org/10.1097/01.fjc.0000211738.63207.c3
Jeong Y, Chaupin DF, Matsushita K, Yamakuchi M, Cameron SJ, Morrell CN, Lowenstein CJ (2009) Aldosterone activates endothelial exocytosis. Proc Natl Acad Sci U S A 106(10):3782–3787. https://doi.org/10.1073/pnas.0804037106
Ishizawa K, Izawa Y, Ito H, Miki C, Miyata K, Fujita Y, Kanematsu Y, Tsuchiya K, Tamaki T, Nishiyama A, Yoshizumi M (2005) Aldosterone stimulates vascular smooth muscle cell proliferation via big mitogen-activated protein kinase 1 activation. Hypertension 46(4):1046–1052. https://doi.org/10.1161/01.HYP.0000172622.51973.f5
Gonzalez-Perez P, Stiefel P (2008) Increased carotid intima-media thickness in hypertensive patients with a high aldosterone/plasma renin activity ratio and elevated aldosterone plasma concentration. J Hypertens 26(7):1499–1500; author reply 1500-1491. https://doi.org/10.1097/HJH.0b013e3282fd6932
Simons PC, Algra A, Bots ML, Grobbee DE, van der Graaf Y (1999) Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients. The SMART study (second manifestations of ARTerial disease). Circulation 100(9):951–957
Grossmann C, Krug AW, Freudinger R, Mildenberger S, Voelker K, Gekle M (2007)Aldosterone-induced EGFR expression: interaction between the human mineralocorticoid receptor and the human EGFR promoter. Am J Physiol Endocrinol Metab 292(6):E1790–E1800. https://doi.org/10.1152/ajpendo.00708.2006
Krug AW, Grossmann C, Schuster C, Freudinger R, Mildenberger S, Govindan MV, Gekle M (2003) Aldosterone stimulates epidermal growth factor receptor expression. J Biol Chem 278(44):43060–43066. https://doi.org/10.1074/jbc.M308134200
Bokemeyer D, Schmitz U, Kramer HJ (2000) Angiotensin II-induced growth of vascular smooth muscle cells requires an Src-dependent activation of the epidermal growth factor receptor. Kidney Int 58(2):549–558. https://doi.org/10.1046/j.1523-1755.2000.t01-1-00201.x
Kagiyama S, Eguchi S, Frank GD, Inagami T, Zhang YC, Phillips MI (2002) Angiotensin II-induced cardiac hypertrophy and hypertension are attenuated by epidermal growth factor receptor antisense. Circulation 106(8):909–912
Lecour S, James RW (2011) When are pro-inflammatory cytokines SAFE in heart failure? Eur Heart J 32(6):680–685. https://doi.org/10.1093/eurheartj/ehq484
Gullestad L, Aukrust P (2005) Review of trials in chronic heart failure showing broad-spectrum anti-inflammatory approaches. Am J Cardiol 95(11A):17C–23C; discussion 38C-40C. https://doi.org/10.1016/j.amjcard.2005.03.008
Lacerda L, Somers S, Opie LH, Lecour S (2009) Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res 84(2):201–208. https://doi.org/10.1093/cvr/cvp274
Lecour S, Suleman N, Deuchar GA, Somers S, Lacerda L, Huisamen B, Opie LH (2005) Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase). Circulation 112(25):3911–3918. https://doi.org/10.1161/circulationaha.105.581058
Anker SD, von Haehling S (2004) Inflammatory mediators in chronic heart failure: an overview. Heart 90(4):464–470
Mann DL (2002) Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 91(11):988–998
White M, Ducharme A, Ibrahim R, Whittom L, Lavoie J, Guertin MC, Racine N, He Y, Yao G, Rouleau JL, Schiffrin EL, Touyz RM (2006) Increased systemic inflammation and oxidative stress in patients with worsening congestive heart failure: improvement after short-term inotropic support. Clin Sci (London, England : 1979) 110(4):483–489. https://doi.org/10.1042/CS20050317
Levine B, Kalman J, Mayer L, Fillit HM, Packer M (1990) Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323(4):236–241. https://doi.org/10.1056/NEJM199007263230405
Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK, Nordoy I, Aass H, Espevik T, Simonsen S, Froland SS, Gullestad L (1999) Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 83(3):376–382
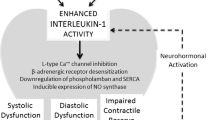
Kiczak L, Paslawska U, Bania J, Ugorski M, Sambor I, Kochman A, Blach J, Chelmonska-Soyta A (2008) Increased expression of interleukin-1beta and its novel splice variant in canine hearts with volume overload. Cytokine 44(3):352–360. https://doi.org/10.1016/j.cyto.2008.10.002
Shioi T, Matsumori A, Kihara Y, Inoko M, Ono K, Iwanaga Y, Yamada T, Iwasaki A, Matsushima K, Sasayama S (1997) Increased expression of interleukin-1 beta and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ Res 81(5):664–671
Stumpf C, Lehner C, Yilmaz A, Daniel WG, Garlichs CD (2003) Decrease of serum levels of the anti-inflammatory cytokine interleukin-10 in patients with advanced chronic heart failure. Clin Sci (London, England : 1979) 105(1):45–50. https://doi.org/10.1042/CS20020359
Aukrust P, Ueland T, Muller F, Andreassen AK, Nordoy I, Aas H, Kjekshus J, Simonsen S, Froland SS, Gullestad L (1998) Elevated circulating levels of C-C chemokines in patients with congestive heart failure. Circulation 97(12):1136–1143
Damas JK, Gullestad L, Ueland T, Solum NO, Simonsen S, Froland SS, Aukrust P (2000) CXC-chemokines, a new group of cytokines in congestive heart failure—possible role of platelets and monocytes. Cardiovasc Res 45(2):428–436
Cappuzzello C, Di Vito L, Melchionna R, Melillo G, Silvestri L, Cesareo E, Crea F, Liuzzo G, Facchiano A, Capogrossi MC, Napolitano M (2011) Increase of plasma IL-9 and decrease of plasma IL-5, IL-7, and IFN-gamma in patients with chronic heart failure. J Transl Med 9:28. https://doi.org/10.1186/1479-5876-9-28
Sanchez-Lazaro IJ, Almenar L, Reganon E, Vila V, Martinez-Dolz L, Martinez-Sales V, Moro J, Aguero J, Ortiz-Martinez V, Salvador A (2008) Inflammatory markers in stable heart failure and their relationship with functional class. Int J Cardiol 129(3):388–393. https://doi.org/10.1016/j.ijcard.2007.07.138
Sato Y, Takatsu Y, Kataoka K, Yamada T, Taniguchi R, Sasayama S, Matsumori A (1999) Serial circulating concentrations of C-reactive protein, interleukin (IL)-4, and IL-6 in patients with acute left heart decompensation. Clin Cardiol 22(12):811–813
Yamaoka M, Yamaguchi S, Okuyama M, Tomoike H (1999)Anti-inflammatory cytokine profile in human heart failure: behavior of interleukin-10 in association with tumor necrosis factor-alpha. Jpn Circ J 63(12):951–956
Paulus WJ (1999) How are cytokines activated in heart failure? Eur J Heart Fail 1(4):309–312
Oikonomou E, Tousoulis D, Siasos G, Zaromitidou M, Papavassiliou AG, Stefanadis C (2011) The role of inflammation in heart failure: new therapeutic approaches. Hell J Cardiol 52(1):30–40
Kalra D, Sivasubramanian N, Mann DL (2002) Angiotensin II induces tumor necrosis factor biosynthesis in the adult mammalian heart through a protein kinase C-dependent pathway. Circulation 105(18):2198–2205
Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA (2002) Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol 283(5):H1802–H1810. https://doi.org/10.1152/ajpheart.01096.2001
Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT (2002)Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am J Pathol 161(5):1773–1781. https://doi.org/10.1016/S0002-9440(10)64454-9
Szabo-Fresnais N, Lefebvre F, Germain A, Fischmeister R, Pomerance M (2010) A new regulation of IL-6 production in adult cardiomyocytes by beta-adrenergic and IL-1 beta receptors and induction of cellular hypertrophy by IL-6 trans-signalling. Cell Signal 22(7):1143–1152. https://doi.org/10.1016/j.cellsig.2010.03.009
Prabhu SD, Chandrasekar B, Murray DR, Freeman GL (2000)Beta-adrenergic blockade in developing heart failure: effects on myocardial inflammatory cytokines, nitric oxide, and remodeling. Circulation 101(17):2103–2109
Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL (1996) Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the studies of left ventricular dysfunction (SOLVD). J Am Coll Cardiol 27(5):1201–1206. https://doi.org/10.1016/0735-1097(95)00589-7
Stumpf C, Lehner C, Raaz D, Yilmaz A, Anger T, Daniel WG, Garlichs CD (2008) Platelets contribute to enhanced MCP-1 levels in patients with chronic heart failure. Heart 94(1):65–69. https://doi.org/10.1136/hrt.2007.115006
Yokoyama T, Nakano M, Bednarczyk JL, McIntyre BW, Entman M, Mann DL (1997) Tumor necrosis factor-alpha provokes a hypertrophic growth response in adult cardiac myocytes. Circulation 95(5):1247–1252
Chrysohoou C, Pitsavos C, Barbetseas J, Kotroyiannis I, Brili S, Vasiliadou K, Papadimitriou L, Stefanadis C (2009) Chronic systemic inflammation accompanies impaired ventricular diastolic function, detected by Doppler imaging, in patients with newly diagnosed systolic heart failure (Hellenic heart failure study). Heart Vessel 24(1):22–26. https://doi.org/10.1007/s00380-008-1080-7
Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL (2001) Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 103(16):2055–2059
Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL (2008) Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation 118(6):625–631. https://doi.org/10.1161/CIRCULATIONAHA.107.759191
Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD (2000) Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 102(25):3060–3067
Pagani FD, Baker LS, Hsi C, Knox M, Fink MP, Visner MS (1992) Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factor-alpha in conscious dogs. J Clin Invest 90(2):389–398. https://doi.org/10.1172/JCI115873
Hosenpud JD, Campbell SM, Mendelson DJ (1989)Interleukin-1-induced myocardial depression in an isolated beating heart preparation. J Heart Transplant 8(6):460–464
Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL (1992) Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science (New York, NY) 257(5068):387–389
Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE (1996) Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 183(3):949–958
Hirota H, Yoshida K, Kishimoto T, Taga T (1995) Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci U S A 92(11):4862–4866
Thaik CM, Calderone A, Takahashi N, Colucci WS (1995)Interleukin-1 beta modulates the growth and phenotype of neonatal rat cardiac myocytes. J Clin Invest 96(2):1093–1099. https://doi.org/10.1172/JCI118095
Siwik DA, Chang DL, Colucci WS (2000)Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res 86(12):1259–1265
Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM (1997) Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res 81(4):627–635
Haudek SB, Taffet GE, Schneider MD, Mann DL (2007) TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J Clin Invest 117(9):2692–2701. https://doi.org/10.1172/JCI29134
Kolattukudy PE, Quach T, Bergese S, Breckenridge S, Hensley J, Altschuld R, Gordillo G, Klenotic S, Orosz C, Parker-Thornburg J (1998) Myocarditis induced by targeted expression of the MCP-1 gene in murine cardiac muscle. Am J Pathol 152(1):101–111
Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O (1995) Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science (New York, NY) 269(5230):1583–1585
Tentolouris C, Tousoulis D, Antoniades C, Bosinakou E, Kotsopoulou M, Trikas A, Toutouzas P, Stefanadis C (2004) Endothelial function and proinflammatory cytokines in patients with ischemic heart disease and dilated cardiomyopathy. Int J Cardiol 94(2–3):301–305. https://doi.org/10.1016/j.ijcard.2003.08.002
Pietri P, Vyssoulis G, Vlachopoulos C, Zervoudaki A, Gialernios T, Aznaouridis K, Stefanadis C (2006) Relationship between low-grade inflammation and arterial stiffness in patients with essential hypertension. J Hypertens 24(11):2231–2238. https://doi.org/10.1097/01.hjh.0000249701.49854.21
Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB (2004)C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol 24(5):969–974. https://doi.org/10.1161/01.ATV.zhq0504.0173
Mahmud A, Feely J (2005) Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 46(5):1118–1122. https://doi.org/10.1161/01.HYP.0000185463.27209.b0
Deisher TA, Haddix TL, Montgomery KF, Pohlman TH, Kaushansky K, Harlan JM (1993) The role of protein kinase C in the induction of VCAM-1 expression on human umbilical vein endothelial cells. FEBS Lett 331(3):285–290
Boring L, Gosling J, Cleary M, Charo IF (1998) Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394(6696):894–897. https://doi.org/10.1038/29788
Zwaka TP, Hombach V, Torzewski J (2001)C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation 103(9):1194–1197
Verma S, Li SH, Badiwala MV, Weisel RD, Fedak PW, Li RK, Dhillon B, Mickle DA (2002) Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation 105(16):1890–1896
Pasceri V, Cheng JS, Willerson JT, Yeh ET (2001) Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation 103(21):2531–2534
Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I (2002) Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation 106(12):1439–1441
Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, Badiwala MV, Mickle DA, Weisel RD, Fedak PW, Stewart DJ, Kutryk MJ (2004)C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation 109(17):2058–2067. https://doi.org/10.1161/01.CIR.0000127577.63323.24
Wang CH, Li SH, Weisel RD, Fedak PW, Dumont AS, Szmitko P, Li RK, Mickle DA, Verma S (2003)C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation 107(13):1783–1790. https://doi.org/10.1161/01.CIR.0000061916.95736.E5
Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, Masson S, Signorini S, Mocarelli P, Hester A, Glazer R, Cohn JN, Val-He FTI (2005)C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation 112(10):1428–1434. https://doi.org/10.1161/CIRCULATIONAHA.104.508465
Yin WH, Chen JW, Jen HL, Chiang MC, Huang WP, Feng AN, Young MS, Lin SJ (2004) Independent prognostic value of elevated high-sensitivity C-reactive protein in chronic heart failure. Am Heart J 147(5):931–938. https://doi.org/10.1016/j.ahj.2003.11.021
Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E (2005)C-reactive protein levels and outcomes after statin therapy. N Engl J Med 352(1):20–28. https://doi.org/10.1056/NEJMoa042378
Khalil ME, Basher AW, Brown EJ Jr, Alhaddad IA (2001) A remarkable medical story: benefits of angiotensin-converting enzyme inhibitors in cardiac patients. J Am Coll Cardiol 37(7):1757–1764
Garg R, Yusuf S (1995) Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative group on ACE inhibitor trials. Jama 273(18):1450–1456
Foody JM, Farrell MH, Krumholz HM (2002)Beta-blocker therapy in heart failure: scientific review. Jama 287(7):883–889
Farrell MH, Foody JM, Krumholz HM (2002)Beta-blockers in heart failure: clinical applications. Jama 287(7):890–897
Groenning BA, Nilsson JC, Sondergaard L, Fritz-Hansen T, Larsson HB, Hildebrandt PR (2000) Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol 36(7):2072–2080
Lage SG, Kopel L, Medeiros CC, Carvalho RT, Creager MA (2002) Angiotensin II contributes to arterial compliance in congestive heart failure. Am J Physiol Heart Circ Physiol 283(4):H1424–H1429. https://doi.org/10.1152/ajpheart.00820.2001
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 128(16):e240–e327. https://doi.org/10.1161/CIR.0b013e31829e8776
Havranek EP, Thomas I, Smith WB, Ponce GA, Bilsker M, Munger MA, Wolf RA (1999)Dose-related beneficial long-term hemodynamic and clinical efficacy of irbesartan in heart failure. J Am Coll Cardiol 33(5):1174–1181
Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, Sharma D, Thiyagarajan B (2000) Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the losartan heart failure survival study ELITE II. Lancet 355(9215):1582–1587
Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358(15):1547–1559. https://doi.org/10.1056/NEJMoa0801317
Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O’Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P (2013) Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369(20):1892–1903. https://doi.org/10.1056/NEJMoa1303154
Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA (2012) Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367(23):2204–2213. https://doi.org/10.1056/NEJMoa1208799
Mahmud A, Feely J (2002) Effect of angiotensin ii receptor blockade on arterial stiffness: beyond blood pressure reduction. Am J Hypertens 15(12):1092–1095
Ozawa Y, Kobori H, Suzaki Y, Navar LG (2007) Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol 292(1):F330–F339. https://doi.org/10.1152/ajprenal.00059.2006
Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Kinoshita M (2000) Angiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin-6 and soluble adhesion molecules in patients with chronic heart failure. J Am Coll Cardiol 35(3):714–721
Adams KF Jr (2004) Pathophysiologic role of the renin-angiotensin-aldosterone and sympathetic nervous systems in heart failure. Am J Health-Syst Pharm 61(Suppl 2):S4–S13
Savoia C, Touyz RM, Amiri F, Schiffrin EL (2008) Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension 51(2):432–439. https://doi.org/10.1161/HYPERTENSIONAHA.107.103267
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone evaluation study investigators. N Engl J Med 341(10):709–717. https://doi.org/10.1056/NEJM199909023411001
Farquharson CA, Struthers AD (2002) Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clin Sci (London, England : 1979) 103(4):425–431. https://doi.org/10.1042/
Albert NM, Yancy CW, Liang L, Zhao X, Hernandez AF, Peterson ED, Cannon CP, Fonarow GC (2009) Use of aldosterone antagonists in heart failure. Jama 302(15):1658–1665. https://doi.org/10.1001/jama.2009.1493
Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B (2011) Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364(1):11–21. https://doi.org/10.1056/NEJMoa1009492
Liu LC, Schutte E, Gansevoort RT, van der Meer P, Voors AA (2015) Finerenone : third-generation mineralocorticoid receptor antagonist for the treatment of heart failure and diabetic kidney disease. Expert Opin Investig Drugs 24(8):1123–1135. https://doi.org/10.1517/13543784.2015.1059819
Pei H, Wang W, Zhao D, Wang L, Su GH, Zhao Z (2018) The use of a novel non-steroidal mineralocorticoid receptor antagonist finerenone for the treatment of chronic heart failure: a systematic review and meta-analysis. Medicine 97(16):e0254. https://doi.org/10.1097/md.0000000000010254
Jhund PS, McMurray JJ (2016) The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart 102(17):1342–1347. https://doi.org/10.1136/heartjnl-2014-306775
Daniels LB, Maisel AS (2007) Natriuretic peptides. J Am Coll Cardiol 50(25):2357–2368. https://doi.org/10.1016/j.jacc.2007.09.021
Nakagawa O, Ogawa Y, Itoh H, Suga S, Komatsu Y, Kishimoto I, Nishino K, Yoshimasa T, Nakao K (1995) Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an "emergency" cardiac hormone against ventricular overload. J Clin Invest 96(3):1280–1287. https://doi.org/10.1172/jci118162
Jensen KT, Carstens J, Pedersen EB (1998) Effect of BNP on renal hemodynamics, tubular function and vasoactive hormones in humans. Am J Phys 274(1 Pt 2):F63–F72
Zellner C, Protter AA, Ko E, Pothireddy MR, DeMarco T, Hutchison SJ, Chou TM, Chatterjee K, Sudhir K (1999) Coronary vasodilator effects of BNP: mechanisms of action in coronary conductance and resistance arteries. Am J Phys 276(3 Pt 2):H1049–H1057
Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Belohlavek J, Bohm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzalez-Medina A, Hagege AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan O, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz-Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS Jr, Silva-Cardoso J, Squire IB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC (2015) Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 131(1):54–61. https://doi.org/10.1161/circulationaha.114.013748
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR (2014)Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371(11):993–1004. https://doi.org/10.1056/NEJMoa1409077
Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E (2019)Angiotensin-Neprilysin inhibition in acute decompensated heart failure. N Engl J Med 380(6):539–548. https://doi.org/10.1056/NEJMoa1812851
Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Shi VC, Lefkowitz MP, McMurray JJV (2017) Angiotensin receptor Neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF Trial. JACC Heart Fail 5(7):471–482. https://doi.org/10.1016/j.jchf.2017.04.013
Redfield MM (2016) Heart failure with preserved ejection fraction. N Engl J Med 375(19):1868–1877. https://doi.org/10.1056/NEJMcp1511175
Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH, Abouzaki NA, Rengel LR, Varma A, Gambill ML, Falcao RA, Voelkel NF, Dinarello CA, Vetrovec GW (2013) Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra remodeling trial (2)(VCU-ART2) pilot study]. Am J Cardiol 111(10):1394–1400. https://doi.org/10.1016/j.amjcard.2013.01.287
Van Tassell BW, Trankle CR, Canada JM, Carbone S, Buckley L, Kadariya D, Del Buono MG, Billingsley H, Wohlford G, Viscusi M, Oddi-Erdle C, Abouzaki NA, Dixon D, Biondi-Zoccai G, Arena R, Abbate A (2018)IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail 11(8):e005036. https://doi.org/10.1161/circheartfailure.118.005036
Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, Abouzaki NA, Dixon D, Kadariya D, Christopher S, Schatz A, Regan J, Viscusi M, Del Buono M, Melchior R, Mankad P, Lu J, Sculthorpe R, Biondi-Zoccai G, Lesnefsky E, Arena R, Abbate A (2017)Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (recently decompensated heart failure Anakinra response trial). Circ Heart Fail 10(11). https://doi.org/10.1161/circheartfailure.117.004373
Ikonomidis I, Lekakis JP, Nikolaou M, Paraskevaidis I, Andreadou I, Kaplanoglou T, Katsimbri P, Skarantavos G, Soucacos PN, Kremastinos DT (2008) Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 117(20):2662–2669. https://doi.org/10.1161/circulationaha.107.731877
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377(12):1119–1131. https://doi.org/10.1056/NEJMoa1707914
Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM (2019)Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 139(10):1289–1299. https://doi.org/10.1161/circulationaha.118.038010
Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ (2019)Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 380(8):752–762. https://doi.org/10.1056/NEJMoa1809798
Bacchiega BC, Bacchiega AB, Usnayo MJ, Bedirian R, Singh G, Pinheiro GD (2017) Interleukin 6 inhibition and coronary artery disease in a high-risk population: a prospective community-based clinical study. J Am Heart Assoc 6(3). https://doi.org/10.1161/jaha.116.005038
Pomerantz BJ, Reznikov LL, Harken AH, Dinarello CA (2001) Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci U S A 98(5):2871–2876. https://doi.org/10.1073/pnas.041611398
Toldo S, Mezzaroma E, O’Brien L, Marchetti C, Seropian IM, Voelkel NF, Van Tassell BW, Dinarello CA, Abbate A (2014)Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Phys Heart Circ Phys 306(7):H1025–H1031. https://doi.org/10.1152/ajpheart.00795.2013
Verma SK, Krishnamurthy P, Barefield D, Singh N, Gupta R, Lambers E, Thal M, Mackie A, Hoxha E, Ramirez V, Qin G, Sadayappan S, Ghosh AK, Kishore R (2012)Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-kappaB. Circulation 126(4):418–429. https://doi.org/10.1161/circulationaha.112.112185
Hoch AZ, Papanek P, Szabo A, Widlansky ME, Schimke JE, Gutterman DD (2011) Association between the female athlete triad and endothelial dysfunction in dancers. Clin J Sport Med 21(2):119–125. https://doi.org/10.1097/JSM.0b013e3182042a9a
Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, Gulliver W, Keeling S, Dutz J, Bessette L, Bissonnette R, Haraoui B (2015) The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 74(3):480–489. https://doi.org/10.1136/annrheumdis-2014-206624
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti TNFTACHFI (2003) Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation 107(25):3133–3140. https://doi.org/10.1161/01.CIR.0000077913.60364.D2
Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T (2004) Targeted anticytokine therapy in patients with chronic heart failure: results of the randomized Etanercept worldwide evaluation (RENEWAL). Circulation 109(13):1594–1602. https://doi.org/10.1161/01.CIR.0000124490.27666.B2
Bozkurt B, Torre-Amione G, Warren MS, Whitmore J, Soran OZ, Feldman AM, Mann DL (2001) Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation 103(8):1044–1047
Mann DL, Bozkurt B, Torre-Amione G, Soran OZ, Sivasubramanian N (2008) Effect of the soluble TNF-antagonist etanercept on tumor necrosis factor bioactivity and stability. Clin Transl Sci 1(2):142–145. https://doi.org/10.1111/j.1752-8062.2008.00013.x
Frishman JI, Edwards CK 3rd, Sonnenberg MG, Kohno T, Cohen AM, Dinarello CA (2000) Tumor necrosis factor (TNF)-alpha-induced interleukin-8 in human blood cultures discriminates neutralization by the p55 and p75 TNF soluble receptors. J Infect Dis 182(6):1722–1730. https://doi.org/10.1086/317605
ten Hove T, van Montfrans C, Peppelenbosch MP, van Deventer SJ (2002) Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn’s disease. Gut 50(2):206–211. https://doi.org/10.1136/gut.50.2.206
Sarzi-Puttini P, Atzeni F, Shoenfeld Y, Ferraccioli G (2005) TNF-alpha, rheumatoid arthritis, and heart failure: a rheumatological dilemma. Autoimmun Rev 4(3):153–161. https://doi.org/10.1016/j.autrev.2004.09.004
Hedayat M, Mahmoudi MJ, Rose NR, Rezaei N (2010) Proinflammatory cytokines in heart failure: double-edged swords. Heart Fail Rev 15(6):543–562. https://doi.org/10.1007/s10741-010-9168-4
Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K (2002) Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat versus Enalapril randomized trial of utility in reducing events (OVERTURE). Circulation 106(8):920–926
Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS (2015)Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol 65(9):867–875. https://doi.org/10.1016/j.jacc.2014.12.026
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group (2002) Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Jama 288(23):2981–2997
Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ (2008) Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 359(23):2417–2428. https://doi.org/10.1056/NEJMoa0806182
Houghton JL (2003)Long-term cardiovascular consequences of diuretics vs calcium channel blockers vs angiotensin-converting enzyme inhibitors. Jama 289(16):2066; author reply 2069-2070. https://doi.org/10.1001/jama.289.16.2066-b
Massie BM (2009) Prevention of heart failure with chlorthalidone in ALLHAT: placing the results into perspective. J Clin Hypertens (Greenwich, Conn) 11(9):462–465. https://doi.org/10.1111/j.1751-7176.2009.00169.x
Funding
No funding was provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grosman-Rimon, L., Billia, F., Wright, E. et al. Neurohormones, inflammatory mediators, and cardiovascular injury in the setting of heart failure. Heart Fail Rev 25, 685–701 (2020). https://doi.org/10.1007/s10741-019-09860-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-019-09860-8