Abstract
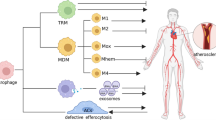
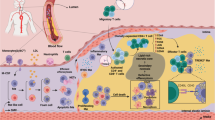
Accumulation of macrophages within the artery wall is an eminent feature of atherosclerotic plaques. Macrophages are influenced by various plaque microenvironmental stimuli, such as oxidized lipids, cytokines, and senescent erythrocytes, and thereby polarize into two main phenotypes called proinflammatory M1 and anti-inflammatory M2 macrophages. In the hemorrhagic zones of atheroma, upon exposure to iron, sequestration of iron by M1 macrophages results in an uncontrolled proinflammatory phenotype impairing wound healing, while M2 macrophages phagocytose both apoptotic cells and senescent erythrocytes. M1 macrophages are prominent phenotype in the unstable plaques, in which plaque shoulder contains macrophages mainly present markers of M1 phenotype, whereas the fibrous cap encompassing the necrotic lipid core content macrophages expressed markers of both M1 and M2 subtypes. The abovementioned findings suggest macrophage modulation as a potent approach for atherosclerosis therapy. Curcumin is a polyphenol dietary derived from turmeric with numerous pharmacological activities. Recent in vitro and in vivo studies have indicated that curcumin exerted lipid-lowering effects, and also can modulate function of different macrophage subsets in various macrophage-involved diseases. The current review aimed to present role of macrophage subtypes in atherosclerosis development and progression, and to understand effect of curcumin on macrophage polarization and foam cell formation in the atherosclerosis lesions. Overall, we would address important targets for macrophage modulation in atherosclerotic plaques.


Similar content being viewed by others
Abbreviations
- ABCA1:
-
ATP-binding cassette transporter
- aP2:
-
Adipocyte protein
- CD163L1:
-
CD163 antigen-like 1
- CERP:
-
Cholesterol efflux regulatory protein
- COX-2:
-
Cyclooxygenase-2
- ERK:
-
Extracellular signal–regulated kinases
- FABPs:
-
Fatty acid-binding proteins
- JNK:
-
c-Jun N-terminal kinase
- iNOS:
-
Inducible nitric-oxide synthase
- MAPK:
-
Mitogen-activated protein kinase
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- SEPP:
-
Selenoprotein P
- GM-CSF:
-
Granulocyte-macrophage colony stimulating factor
- IFN γ:
-
Interferon gamma
- IL:
-
Interleukin
- LDL-C:
-
Low-density lipoprotein cholesterol
- LPS:
-
Lipopolysaccharide
- LXR-α:
-
Liver X receptor alpha
- M-CSF:
-
Macrophage colony-stimulating factor
- NFE2L2:
-
Nuclear factor (erythroid-derived 2)-like 2
- Ox-LDL:
-
Oxidized-LDL
- SR:
-
Scavenger receptors
- TNF:
-
Tumor necrosis factor
- LPS:
-
Llipopolysaccharide
- PI3K:
-
Phosphoinositide 3-kinase
- PPAR-γ:
-
Peroxisome proliferator-activated receptor gamma
- RCT:
-
Reverse cholesterol transport
- ROS:
-
Reactive oxygen species
- S1P:
-
Sphingosine-1-phosphate
- Th1:
-
T helper 1
- TLRs:
-
Toll-like receptors
- TNF-α:
-
Tumor necrosis factor alpha
- TGF-β:
-
Transforming growth factor β
- VSMCs:
-
Vascular smooth muscle cells
References
Moss JW, Ramji DP (2016) Nutraceutical therapies for atherosclerosis. Nat Rev Cardiol 13:513–532
Lusis AJ (2000) Atherosclerosis. Nature 407(6801):233–241
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340(2):115–126
Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M (1995) Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci 92(18):8264–8268
Peiser L, Mukhopadhyay S, Gordon S (2002) Scavenger receptors in innate immunity. Curr Opin Immunol 14(1):123–128
Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20(1):197–216
Tabas I (2005) Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol 25(11):2255–2264
Geissmann F, Jung S, Littman DR (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19(1):71–82
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5(12):953–964
Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE (2011) Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332(6035):1284–1288
Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LMS, Smyth D, Zavitz CCJ, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK (2013) Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 19(9):1166–1172
Jenkins SJ et al (2013) IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med 210:2477. https://doi.org/10.1084/jem.20121999
Biswas SK, Chittezhath M, Shalova IN, Lim JY (2012) Macrophage polarization and plasticity in health and disease. Immunol Res 53(1–3):11–24
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11(11):723–737
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3(1):23–35
Verreck FA et al (2004) Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco) bacteria. Proc Natl Acad Sci U S A 101(13):4560–4565
Mosser DM (2003) The many faces of macrophage activation. J Leukoc Biol 73(2):209–212
Mantovani A et al (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25(12):677–686
Zizzo G, Hilliard BA, Monestier M, Cohen PL (2012) Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol 189(7):3508–3520
Chinetti-Gbaguidi G et al (2011) Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ Res 108:985–995. https://doi.org/10.1161/CIRCRESAHA.110.233775
Jetten N, Verbruggen S, Gijbels MJ, Post MJ, de Winther MPJ, Donners MMPC (2014) Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17(1):109–118
Tabas I (2010) Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol 10(1):36–46
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969
Porcheray F, Viaud S, Rimaniol AC, Léone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G (2005) Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol 142(3):481–489
Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG (2011) Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22(2):317–326
Chinetti-Gbaguidi G, Colin S, Staels B (2015) Macrophage subsets in atherosclerosis. Nat Rev Cardiol 12(1):10–17
Stöger JL, Gijbels MJJ, van der Velden S, Manca M, van der Loos CM, Biessen EAL, Daemen MJAP, Lutgens E, de Winther MPJ (2012) Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 225(2):461–468
Cho KY, Miyoshi H, Kuroda S, Yasuda H, Kamiyama K, Nakagawara J, Takigami M, Kondo T, Atsumi T (2013) The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in the carotid artery. J Stroke Cerebrovasc Dis 22(7):910–918
Shaikh S, Brittenden J, Lahiri R, Brown PAJ, Thies F, Wilson HM (2012) Macrophage subtypes in symptomatic carotid artery and femoral artery plaques. Eur J Vasc Endovasc Surg 44(5):491–497
Barlis P, Serruys PW, DeVries A, Regar E (2008) Optical coherence tomography assessment of vulnerable plaque rupture: predilection for the plaque ‘shoulder’. Eur Heart J 29(16):2023–2023
Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA (2001) Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med 194(6):809–822
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464(7293):1357–1361
Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI (2009) Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4–and spleen tyrosine kinase–dependent activation of NADPH oxidase 2. Circ Res 104(2):210–218
Van Tits L et al (2011) Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Krüppel-like factor 2. Atherosclerosis 214(2):345–349
Hirose K, Iwabuchi K, Shimada K, Kiyanagi T, Iwahara C, Nakayama H, Daida H (2011) Different responses to oxidized low-density lipoproteins in human polarized macrophages. Lipids Health Dis 10(1):1
Fang L, Harkewicz R, Hartvigsen K, Wiesner P, Choi SH, Almazan F, Pattison J, Deer E, Sayaphupha T, Dennis EA, Witztum JL, Tsimikas S, Miller YI (2010) Oxidized cholesteryl esters and phospholipids in zebrafish larvae fed a high cholesterol diet macrophage binding and activation. J Biol Chem 285(42):32343–32351
Sottero B, Gamba P, Longhi M, Robbesyn F, Abuja PM, Schaur RJ, Poli G, Leonarduzzi G (2005) Expression and synthesis of TGFβ1 is induced in macrophages by 9-oxononanoyl cholesterol, a major cholesteryl ester oxidation product. Biofactors 24(1–4):209–216
Mitchell PL, McLeod RS (2008) Conjugated linoleic acid and atherosclerosis: studies in animal models. Biochem Cell Biol 86(4):293–301
McCarthy C, Duffy MM, Mooney D, James WG, Griffin MD, Fitzgerald DJ, Belton O (2013) IL-10 mediates the immunoregulatory response in conjugated linoleic acid-induced regression of atherosclerosis. FASEB J 27(2):499–510
Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA (2011) HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci 108(17):7166–7171
Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC (2008) Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res 102(8):950–958
Titos E, Rius B, González-Périz A, López-Vicario C, Morán-Salvador E, Martínez-Clemente M, Arroyo V, Clària J (2011) Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol 187(10):5408–5418
Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M (2009) Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med 206(1):15–23
Wolfs IM, Donners MM, de Winther MP (2011) Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb Haemost 105(05):763–771
Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G (2007) PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6(2):137–143
Waldo SW, Li Y, Buono C, Zhao B, Billings EM, Chang J, Kruth HS (2008) Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol 172(4):1112–1126
Brochériou I, Maouche S, Durand H, Braunersreuther V, le Naour G, Gratchev A, Koskas F, Mach F, Kzhyshkowska J, Ninio E (2011) Antagonistic regulation of macrophage phenotype by M-CSF and GM-CSF: implication in atherosclerosis. Atherosclerosis 214(2):316–324
Plenz G, Koenig C, Severs NJ, Robenek H (1997) Smooth muscle cells express granulocyte-macrophage colony-stimulating factor in the undiseased and atherosclerotic human coronary artery. Arterioscler Thromb Vasc Biol 17(11):2489–2499
Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R (2003) Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 349(24):2316–2325
Kockx MM, Cromheeke KM, Knaapen MWM, Bosmans JM, de Meyer GRY, Herman AG, Bult H (2003) Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler Thromb Vasc Biol 23(3):440–446
Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkötter C, Scharffetter-Kochanek K (2011) An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 121(3):985–997
Bories G et al (2013) Liver X receptor (LXR) activation stimulates iron export in human alternative macrophages. Circ Res 113:1196–1205. https://doi.org/10.1161/CIRCRESAHA.113.301656
Nielsen MJ, Møller HJ, Moestrup SK (2010) Hemoglobin and heme scavenger receptors. Antioxid Redox Signal 12(2):261–273
Finn AV, Nakano M, Polavarapu R, Karmali V, Saeed O, Zhao XQ, Yazdani S, Otsuka F, Davis T, Habib A, Narula J, Kolodgie FD, Virmani R (2012) Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol 59(2):166–177
Vivo SBI (2004) Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis. Circ Res 94:119–126
Landis RC, Philippidis P, Domin J, Boyle JJ, Haskard DO (2013) Haptoglobin genotype-dependent anti-inflammatory signaling in CD163. Int J Inflamm 2013:1–7
Strimpakos AS, Sharma RA (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10(3):511–546
Aggarwal BB (2010) Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr 30:173–199
Epstein J, Sanderson IR, MacDonald TT (2010) Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr 103(11):1545–1557
Shehzad A, Ha T, Subhan F, Lee YS (2011) New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr 50(3):151–161
Momtazi AA et al (2016) Curcumin as a MicroRNA regulator in cancer: a review. Rev Physiol Biochem Pharmacol 171:1–38
Momtazi AA, Derosa G, Maffioli P, Banach M, Sahebkar A (2016) Role of microRNAs in the therapeutic effects of curcumin in non-cancer diseases. Mol Diagn Ther 20(4):335–345
Aeineh N et al (2018) Glutathione conjugated polyethylenimine on the surface of Fe3O4 magnetic nanoparticles as a theranostic agent for targeted and controlled curcumin delivery. J Biomater Sci Polym Ed 29(10):1109–1125
Momtazi-Borojeni AA et al (2017) Curcumin: a natural modulator of immune cells in systemic lupus erythematosus. Autoimmun Rev 17(2):125–135
Schaffer M, Schaffer PM, Zidan J, Sela GB (2011) Curcuma as a functional food in the control of cancer and inflammation. Curr Opin Clin Nutr Metab Care 14(6):588–597
Zhong Y, Liu T, Guo Z (2012) Curcumin inhibits ox-LDL-induced MCP-1 expression by suppressing the p38MAPK and NF-κB pathways in rat vascular smooth muscle cells. Inflamm Res 61(1):61–67
Abdollahi E et al (2017) Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: a nature-made jack-of-all-trades? J Cell Physiol
Momtazi-Borojeni AA et al Curcumin in advancing treatment for gynecological cancers with developed drug- and radiotherapy-associated resistance. Springer, Berlin, pp 1–23
Jang E-M, Choi MS, Jung UJ, Kim MJ, Kim HJ, Jeon SM, Shin SK, Seong CN, Lee MK (2008) Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat–fed hamsters. Metab Clin Exp 57(11):1576–1583
Kim M, Kim Y (2010) Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr Res Pract 4(3):191–195
Shin SK, Ha TY, McGregor RA, Choi MS (2011) Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol Nutr Food Res 55(12):1829–1840
Wang MY ( 2012) Spice up your lipids: the effects of curcumin on lipids in humans. Nutr Bytes 16(1)
Yang YS, Su YF, Yang HW, Lee YH, Chou JI, Ueng KC (2014) Lipid-lowering effects of curcumin in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled trial. Phytother Res 28(12):1770–1777
Na LX, Li Y, Pan HZ, Zhou XL, Sun DJ, Meng M, Li XX, Sun CH (2013) Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol Nutr Food Res 57(9):1569–1577
Panahi Y, Khalili N, Hosseini MS, Abbasinazari M, Sahebkar A (2014) Lipid-modifying effects of adjunctive therapy with curcuminoids–piperine combination in patients with metabolic syndrome: results of a randomized controlled trial. Complement Ther Med 22(5):851–857
Panahi Y, Ahmadi Y, Teymouri M, Johnston TP, Sahebkar A (2018) Curcumin as a potential candidate for treating hyperlipidemia: a review of cellular and metabolic mechanisms. J Cell Physiol 233(1):141–152
Hasan S et al (2014) Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis 232(1):40–51
Zhao JF, Ching LC, Huang YC, Chen CY, Chiang AN, Kou YR, Shyue SK, Lee TS (2012) Molecular mechanism of curcumin on the suppression of cholesterol accumulation in macrophage foam cells and atherosclerosis. Mol Nutr Food Res 56(5):691–701
Quiles JL et al (2002) Curcuma longa extract supplementation reduces oxidative stress and attenuates aortic fatty streak development in rabbits. Arterioscler Thromb Vasc Biol 22(7):1225–1231
Ramırez-Tortosa M et al (1999) Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis 147(2):371–378
Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MRF (2001) Lack of macrophage fatty-acid–binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med 7(6):699–705
Fu Y, Luo N, Lopes-Virella MF, Garvey WT (2002) The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis 165(2):259–269
Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MRF, Hotamisligil G̈S (2002) Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler Thromb Vasc Biol 22(10):1686–1691
Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS (2005) The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem 280(13):12888–12895
Zingg JM, Hasan ST, Cowan D, Ricciarelli R, Azzi A, Meydani M (2012) Regulatory effects of curcumin on lipid accumulation in monocytes/macrophages. J Cell Biochem 113(3):833–840
Kou MC, Chiou SY, Weng CY, Wang L, Ho CT, Wu MJ (2013) Curcuminoids distinctly exhibit antioxidant activities and regulate expression of scavenger receptors and heme oxygenase-1. Mol Nutr Food Res 57(9):1598–1610
Chen F-Y, Zhou J, Guo N, Ma WG, Huang X, Wang H, Yuan ZY (2015) Curcumin retunes cholesterol transport homeostasis and inflammation response in M1 macrophage to prevent atherosclerosis. Biochem Biophys Res Commun 467(4):872–878
Zingg JM, Hasan ST, Nakagawa K, Canepa E, Ricciarelli R, Villacorta L, Azzi A, Meydani M (2017) Modulation of cAMP levels by high-fat diet and curcumin and regulatory effects on CD36/FAT scavenger receptor/fatty acids transporter gene expression. Biofactors 43(1):42–53
Zhou Y, Zhang T, Wang X, Wei X, Chen Y, Guo L, Zhang J, Wang C (2015) Curcumin modulates macrophage polarization through the inhibition of the toll-like receptor 4 expression and its signaling pathways. Cell Physiol Biochem 36(2):631–641
Youn HS, Saitoh SI, Miyake K, Hwang DH (2006) Inhibition of homodimerization of toll-like receptor 4 by curcumin. Biochem Pharmacol 72(1):62–69
Chen Y-R, Tan T-H (1998) Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 17(2):173–178
Jacob A, Wu R, Zhou M, Wang P (2007) Mechanism of the anti-inflammatory effect of curcumin: PPAR-γ activation. PPAR Res 2007:1–5
Chen F, Guo N, Cao G, Zhou J, Yuan Z (2014) Molecular analysis of curcumin-induced polarization of murine RAW264. 7 macrophages. J Cardiovasc Pharmacol 63(6):544–552
Gao S, Zhou J, Liu N, Wang L, Gao Q, Wu Y, Zhao Q, Liu P, Wang S, Liu Y, Guo N, Shen Y, Wu Y, Yuan Z (2015) Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J Mol Cell Cardiol 85:131–139
Li B et al (2017) Curcumin attenuates titanium particle-induced inflammation by regulating macrophage polarization in vitro and in vivo. Front Immunol 8:55
Medbury H, Tarran SL, Guiffre AK, Williams MM, Lam TH, Vicaretti M, Fletcher JP (2008) Monocytes contribute to the atherosclerotic cap by transformation into fibrocytes. Int Angiol 27(2):114–123
Chistiakov DA, Bobryshev YV, Nikiforov NG, Elizova NV, Sobenin IA, Orekhov AN (2015) Macrophage phenotypic plasticity in atherosclerosis: the associated features and the peculiarities of the expression of inflammatory genes. Int J Cardiol 184:436–445
Bosisio D et al (2002) Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-γ: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood 99(9):3427–3431
Meng Z, Yan C, Deng Q, Gao DF, Niu XL (2013) Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol Sin 34(7):901–911
Cao J, Han Z, Tian L, Chen K, Fan Y, Ye B, Huang W, Wang C, Huang Z (2014) Curcumin inhibits EMMPRIN and MMP-9 expression through AMPK-MAPK and PKC signaling in PMA induced macrophages. J Transl Med 12(1):266
Gradišar H, Keber MM, Pristovšek P, Jerala R (2007) MD-2 as the target of curcumin in the inhibition of response to LPS. J Leukoc Biol 82(4):968–974
Kong F et al (2016) Curcumin represses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB and P2X7R signaling in PMA-induced macrophages. Front Pharmacol 7:369
Lee K-H, Chow YL, Sharmili V, Abas F, Alitheen NBM, Shaari K, Israf DA, Lajis NH, Syahida A (2012) BDMC33, a curcumin derivative suppresses inflammatory responses in macrophage-like cellular system: role of inhibition in NF-κB and MAPK signaling pathways. Int J Mol Sci 13(3):2985–3008
Bai X, Oberley-Deegan RE, Bai A, Ovrutsky AR, Kinney WH, Weaver M, Zhang G, Honda JR, Chan ED (2016) Curcumin enhances human macrophage control of Mycobacterium tuberculosis infection. Respirology 21(5):951–957
Li H, Sun B (2007) Toll-like receptor 4 in atherosclerosis. J Cell Mol Med 11(1):88–95
Meng Z, Yan C, Deng Q, Dong X, Duan ZM, Gao DF, Niu XL (2013) Oxidized low-density lipoprotein induces inflammatory responses in cultured human mast cells via toll-like receptor 4. Cell Physiol Biochem 31(6):842–853
Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M (2004) Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A 101(29):10679–10684
Björkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW (2004) Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med 10(4):416–421
Acknowledgments
The authors would like to say special thanks to the cooperation of Pars Advanced and Minimally Invasive Medical Manners Research Center - Pars Hospital, Nanotechnology Research Center, and Department of Medical Biotechnology of Mashhad University of Medical Sciences for their kindness.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no direct conflict of interests related to the content of this review.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Momtazi-Borojeni, A.A., Abdollahi, E., Nikfar, B. et al. Curcumin as a potential modulator of M1 and M2 macrophages: new insights in atherosclerosis therapy. Heart Fail Rev 24, 399–409 (2019). https://doi.org/10.1007/s10741-018-09764-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-018-09764-z