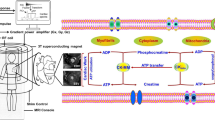
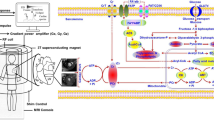
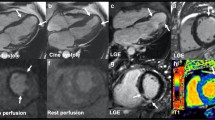
Abstract
Cardiac magnetic resonance spectroscopy (MRS) is a noninvasive method to assess by-products of myocardial metabolism. Recent developments in shorter scan protocols and more powerful field strengths have created interest in utilizing this technology in studying and characterizing the metabolic derangements in heart failure patients. Our lack of understanding in heart failure could be greatly enhanced by identifying the metabolic changes and eventually modifying metabolic substrate to achieve improved cardiac mechanics with the aid of this technology. However, there are several impediments for the widespread applicability of this technology. This review discusses the principals of human cardiac MRS and literature pertaining to use of MRS in patients with cardiomyopathy.





Similar content being viewed by others
References
Ackerman JJ, Grove TH, Wong GG, Gadian DG, Radda GK (1980) Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature 283:167–170
Garlick PB, Radda GK, Seeley PJ, Chance B (1977) Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun 74:1256–1262
Ackerman JJ, Gadian DG, Radda GK, Wong GG (1981) Observation of 1H NMR signals with receiver coils tuned for other nuclides. J Magn Reson (1969) 42:498–500
Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, Wright GA, Connelly KA (2016) Hyperpolarized 13C metabolic MRI of the human heart: novelty and significance. Circ Res 119:1177–1182
Granot J (1986) Selected volume excitation using stimulated echoes (VEST). Applications to spatially localized spectroscopy and imaging. J Magn Reson (1969) 70:488–492
Hamilton G, Middleton MS, Bydder M, Yokoo T, Schwimmer JB, Kono Y, Patton HM, Lavine JE, Sirlin CB (2009) Effect of press and steam sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging 30:145–152
Acorn N (2000) NUTS NMR utility transform software. Acorn NMR, Livermore
Naressi A, Couturier C, Devos J, Janssen M, Mangeat C, De Beer R, Graveron-Demilly D (2001) Java-based graphical user interface for the MRUI quantitation package. MAGMA 12:141–152
Turner CE, Russell BR, Gant N (2015) Comparative quantification of dietary supplemented neural creatine concentrations with 1 H-MRS peak fitting and basis spectrum methods. Magn Reson Imaging 33:1163–1167
Osbakken M, Mitchell MD, Zhang D, Mayevsky A, Chance B (1991) In vivo correlation of myocardial metabolism, perfusion, and mechanical function during increased cardiac work. Cardiovasc Res 25:749–756
Lamb HJ, Beyerbacht HP, Ouwerkerk R, Doornbos J, Pluim BM, van der Wall EE, van der Laarse A, de Roos A (1997) Metabolic response of normal human myocardium to high-dose atropine-dobutamine stress studied by 31P-MRS. Circulation 96:2969–2977
Pluim BM, Lamb HJ, Kayser HW, Leujes F, Beyerbacht HP, Zwinderman AH, van der Laarse A, Vliegen HW, de Roos A, van der Wall EE (1998) Functional and metabolic evaluation of the athlete’s heart by magnetic resonance imaging and dobutamine stress magnetic resonance spectroscopy. Circulation 97:666–672
Clarke K, O’Connor AJ, Willis RJ (1987) Temporal relation between energy metabolism and myocardial function during ischemia and reperfusion. Am J Phys 253:H412–H421
Weiss RG, Bottomley PA, Hardy CJ, Gerstenblith G (1990) Regional myocardial metabolism of high-energy phosphates during isometric exercise in patients with coronary artery disease. N Engl J Med 323:1593–1600
Johnson BD, Shaw LJ, Buchthal SD, Merz CNB, Kim H-W, Scott KN, Doyle M, Olson MB, Pepine CJ, den Hollander J (2004) Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease results from the National Institutes of Health–National Heart, Lung, and Blood Institute–Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 109:2993–2999
Buchthal SD, den Hollander JA, Merz CNB, Rogers WJ, Pepine CJ, Reichek N, Sharaf BL, Reis S, Kelsey SF, Pohost GM (2000) Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med 342:829–835
Yabe T, Mitsunami K, Inubushi T, Kinoshita M (1995) Quantitative measurements of cardiac phosphorus metabolites in coronary artery disease by 31P magnetic resonance spectroscopy. Circulation 92:15–23
Hetherington HP, Luney DJ, Thomas Vaughan J, Pan JW, Ponder SL, Tschendel O, Twieg DB, Pohost GM (1995) 3D 31P spectroscopic imaging of the human heart at 4.1 T. Magn Reson Med 33:427–431
Bottomley PA, Weiss RG (1998) Non-invasive magnetic-resonance detection of creatine depletion in non-viable infarcted myocardium. Lancet 351:714–718
Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G (1991) Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J 122:795–801
Nakae I, Mitsunami K, Matsuo S, Matsumoto T, Morikawa S, Inubushi T, Koh T, Horie M (2004) Assessment of myocardial creatine concentration in dysfunctional human heart by proton magnetic resonance spectroscopy. Magnetic resonance in medical sciences : MRMS : an official journal of Japan Society of Magnetic Resonance in Medicine 3:19–25
Senturk T, Tutuncu A, Ozdemir B, Ozdabakoglu O, Aydin S, Baran I, Gullulu S, Savci V, Aydinlar A (2010) Serum choline levels in patients with stable angina and acute coronary syndromes: relation to the severity of coronary artery disease. Coron Artery Dis 21:466–471
Levelt E, Ntusi N, Mahmod M, Wainwright C, Piechnik S, Francis J, Davis A, Schneider J, Leeson P, Karamitsos T (2014) 127 early manifestations of diabetic cardiomyopathy assessed by cardiac magnetic resonance imaging and spectroscopy. Heart 100:A73–A74
Metzler B, Schocke MF, Steinboeck P, Wolf C, Judmaier W, Lechleitner M, Lukas P, Pachinger O (2002) Decreased high-energy phosphate ratios in the myocardium of men with diabetes mellitus type I. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 4:493–502
van der Meer R, Rijzewijk L, Smit J, Diamant M, Bax J, Hammer S, Romijn J, de Roos A, Lamb H (2008) Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. Myocardial Steatosis and Left Ventricular Function in Type 2 Diabetes Mellitus 137
McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS (2007) Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 116:1170–1175
Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K (2003) Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 107:3040–3046
Snel M, Hammer S, Lamb HJ, Jazet IM, Van der Meer RW, Pijl H, Meinders AE, Romijn JA (2011) Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. The Effects of a Very Low Calorie Diet and Exercise in Obese Type 2 Diabetes Mellitus Patients 52:107
Hammer S, Snel M, Lamb HJ, Jazet IM, van der Meer RW, Pijl H, Meinders EA, Romijn JA, de Roos A, Smit JW (2008) Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol 52:1006–1012
van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA, Bax JJ, de Roos A, Kamp O, Paulus WJ (2009) Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation 119:2069–2077
Beer M, Seyfarth T, Sandstede J, Landschütz W, Lipke C, Köstler H, von Kienlin M, Harre K, Hahn D, Neubauer S (2002) Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol 40:1267–1274
Heyne J-P, Rzanny R, Hansch A, Leder U, Reichenbach J, Kaiser W (2006) 31P-MR spectroscopic imaging in hypertensive heart disease. Eur Radiol 16:1796–1802
Lamb HJ, Beyerbacht HP, Van Der Laarse A, Stoel BC, Doornbos J, van der Wall EE, de Roos A (1999) Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation 99:2261–2267
Conway MA, Bottomley PA, Ouwerkerk R, Radda GK, Rajagopalan B (1998) Mitral regurgitation impaired systolic function, eccentric hypertrophy, and increased severity are linked to lower phosphocreatine/ATP ratios in humans. Circulation 97:1716–1723
Neubauer S, Horn M, Pabst T, Harre K, Stromer H, Bertsch G, Sandstede J, Ertl G, Hahn D, Kochsiek K (1997) Cardiac high-energy phosphate metabolism in patients with aortic valve disease assessed by 31P-magnetic resonance spectroscopy. Journal of investigative medicine : the official publication of the American Federation for Clinical Research 45:453–462
Conway MA, Allis J, Ouwerkerk R, Niioka T, Rajagopalan B, Radda GK (1991) Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 338:973–976
Beyerbacht HP, Lamb HJ, van Der Laarse A, Vliegen HW, Leujes F, Hazekamp MG, de Roos A, van Der Wall EE (2001) Aortic valve replacement in patients with aortic valve stenosis improves myocardial metabolism and diastolic function. Radiology 219:637–643
Mahmod M, Francis JM, Pal N, Lewis A, Dass S, De Silva R, Petrou M, Sayeed R, Westaby S, Robson MD (2014) Myocardial perfusion and oxygenation are impaired during stress in severe aortic stenosis and correlate with impaired energetics and subclinical left ventricular dysfunction. J Cardiovasc Magn Reson 16:1
Ormerod JO, Frenneaux MP (2016) Sherrid MV. Myocardial energy depletion and dynamic systolic dysfunction in hypertrophic cardiomyopathy, Nature Reviews Cardiology
Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, McKenna WJ, Östman-Smith I, Clarke K, Watkins H (2003) Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol 41:1776–1782
Jung W-I, Sieverding L, Breuer J, Hoess T, Widmaier S, Schmidt O, Bunse M, van Erckelens F, Apitz J, Lutz O (1998) 31P NMR spectroscopy detects metabolic abnormalities in asymptomatic patients with hypertrophic cardiomyopathy. Circulation 97:2536–2542
Abraham MR, Bottomley PA, Dimaano VL, Pinheiro A, Steinberg A, Traill TA, Abraham TP, Weiss RG (2013) Creatine kinase adenosine triphosphate and phosphocreatine energy supply in a single kindred of patients with hypertrophic cardiomyopathy. Am J Cardiol 112:861–866
Ewijk PA, Schrauwen-Hinderling VB, Bekkers SC, Glatz JF, Wildberger JE, Kooi ME (2015) MRS: a noninvasive window into cardiac metabolism. NMR Biomed 28:747–766
Dass S, Cochlin LE, Suttie JJ, Holloway CJ, Rider OJ, Carden L, Tyler DJ, Karamitsos TD, Clarke K, Neubauer S (2015) Exacerbation of cardiac energetic impairment during exercise in hypertrophic cardiomyopathy: a potential mechanism for diastolic dysfunction. Eur Heart J ehv120
Crilley JG, Boehm EA, Rajagopalan B, Blamire AM, Styles P, Muntoni F, Hilton-Jones D, Clarke K (2000) Magnetic resonance spectroscopy evidence of abnormal cardiac energetics in Xp21 muscular dystrophy. J Am Coll Cardiol 36:1953–1958
Schocke MF, Martinek M, Kremser C, Wolf C, Steinboeck P, Lechleitner M, Jaschke W, Pachinger O, Metzler B (2003) 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors improve myocardial high-energy phosphate metabolism in men. J Cardiovasc Magn Reson 5:595–602
Schocke MF, Zoller H, Vogel W, Wolf C, Kremser C, Steinboeck P, Poelzl G, Pachinger O, Jaschke WR, Metzler B (2004) Cardiac phosphorus-31 two-dimensional chemical shift imaging in patients with hereditary hemochromatosis. Magn Reson Imaging 22:515–521
Petritsch B, Köstler H, Machann W, Horn M, Weng A, Goltz J, Hahn D, Niemann M, Weidemann F, Wanner C (2012) Non-invasive determination of myocardial lipid content in Fabry disease by 1H-MR spectroscopy. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren 184:1020–1025
Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, Mader H, Kromer EP, Riegger GA, Lackner K et al (1992) 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 86:1810–1818
Fragasso G, Perseghin G, De Cobelli F, Esposito A, Palloshi A, Lattuada G, Scifo P, Calori G, Del Maschio A, Margonato A (2006) Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J 27:942–948
Beer M, Wagner D, Myers J, Sandstede J, Köstler H, Hahn D, Neubauer S, Dubach P (2008) Effects of exercise training on myocardial energy metabolism and ventricular function assessed by quantitative phosphorus-31 magnetic resonance spectroscopy and magnetic resonance imaging in dilated cardiomyopathy. J Am Coll Cardiol 51:1883–1891
Spoladore R, Fragasso G, Perseghin G, De Cobelli F, Esposito A, Maranta F, Calori G, Locatelli M, Lattuada G, Scifo P (2013) Beneficial effects of beta-blockers on left ventricular function and cellular energy reserve in patients with heart failure. Fundam Clin Pharmacol 27:455–464
Wittlinger T, Voigtlander T, Kreitner KF, Kalden P, Genth-Zotz S, Darius H, Thelen M, Meyer J (1998) 31P-MR spectroscopy in human end-stage heart failure during therapy with recombinant human growth hormone. MAGMA 6:171–172
van der Meer RW, Hammer S, Smit JW, Frölich M, Bax JJ, Diamant M, Rijzewijk LJ, de Roos A, Romijn JA, Lamb HJ (2007) Short-term caloric restriction induces accumulation of myocardial triglycerides and decreases left ventricular diastolic function in healthy subjects. Diabetes 56:2849–2853
Holloway CJ, Cochlin LE, Emmanuel Y, Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight NS, Saxby BK (2011) A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am J Clin Nutr 93:748–755
Tyler DJ, Hudsmith LE, Clarke K, Neubauer S, Robson MD (2008) A comparison of cardiac 31P MRS at 1.5 and 3 T. NMR Biomed 21:793–798
Clarke WT, Robson MD, Rodgers CT (2015) Bloch-Siegert B1+-mapping for human cardiac 31P-MRS at 7 Tesla. Magn Reson Med
Gillinder L, Goo SY, Cowin G, Strudwick M, van der Geest RJ, Wang WY, Ng AC (2015) Quantification of intramyocardial metabolites by proton magnetic resonance spectroscopy. Front Cardiovasc Med 2
Bastiaansen JA, Cheng T, Lei H, Gruetter R, Comment A (2015) Direct noninvasive estimation of myocardial tricarboxylic acid cycle flux in vivo using hyperpolarized 13 C magnetic resonance. J Mol Cell Cardiol 87:129–137
Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, Wright GA, Connelly KA (2016) Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res CIRCRESAHA.116.309769
Schroeder MA, Lau AZ, Chen AP, Gu Y, Nagendran J, Barry J, Hu X, Dyck JR, Tyler DJ, Clarke K (2013) Hyperpolarized 13C magnetic resonance reveals early- and late-onset changes to in vivo pyruvate metabolism in the failing heart. Eur J Heart Fail 15:130–140
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Qureshi reports receiving honorarium/consultation fee from Medicure.
Rights and permissions
About this article
Cite this article
Qureshi, W.T., Nasir, U.b. Principals and clinical applications of magnetic resonance cardiac spectroscopy in heart failure. Heart Fail Rev 22, 491–499 (2017). https://doi.org/10.1007/s10741-017-9611-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-017-9611-x