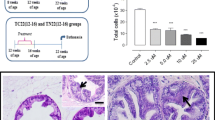
Abstract
Prostate cancer (PCa) is the second cause of cancer death among men worldwide. Several processes are involved in the development and progression of PCa such as angiogenesis, inflammation and oxidative stress. The present study investigated the effect of short- or long-term Tempol treatment at different stages of prostate adenocarcinoma progression, focusing on angiogenic, proliferative, and stromal remodeling processes in TRAMP mice. The dorsolateral lobe of the prostate of TRAMP mice were evaluated at two different stages of PCa progression; early and late stages. Early stage was again divided into, short- or long-term. 50 mg/kg Tempol dose was administered orally. The results demonstrated that Tempol mitigated the prostate histopathological lesion progressions in the TRAMP mice in all treated groups. However, Tempol increased molecules involved in the angiogenic process such as CD31 and VEGFR2 relative frequencies, particularly in long-term treatment. In addition, Tempol upregulated molecule levels involved in angiogenesis and stromal remodeling process VEGF, TGF-β1, VE-cadherin and vimentin, particularly, in T8-16 group. Thus, it was concluded that Tempol treatment delayed prostatic lesion progression in the dorsolateral lobe of the TRAMP mice. However, Tempol also led to pro-angiogenic effects and glandular stromal microenvironment imbalance, especially, in the long-term treatment.







Similar content being viewed by others
References
Barron DA et al (2012) The reactive stroma microenvironment and prostate cancer progression. Endocrine-related cancer 19(6):R187–R204
Battisti V et al (2011) Oxidative stress and antioxidant status in prostate cancer patients: relation to Gleason score, treatment and bone metastasis. Biomed Pharmacother 65(7):516–524
Bavik C et al (2006) The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res 66(2):794–802
Berman-Booty LD (2012) A review of the existing grading schemes and a proposal for a modified grading scheme for prostatic lesions in TRAMP mice. Toxicologic Pathol 10(1):5–17
Cardano M et al (2020) Targeting proliferating cell nuclear antigen (PCNA) as an effective strategy to inhibit tumor cell proliferation. Curr Cancer Drug Targ 20(4):240–252
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307
Condon MS (2005) The role of the stromal microenvironment in prostate cancer. Seminars in cancer biology. Academic, pp 132–137.
Cunha GR et al (2003) Role of the stromal microenvironment in carcinogenesis of the prostate. Prost Ren Cancer, Benign Prost Hyperplasia, Erect Dysfunct Basic Res 107(1):1–10
De Nunzio C et al (2012) The correlation between metabolic syndrome and prostatic diseases. Eur Urol 61(3):560–570
Gelanin DP (2009) A systematic review of human antioxidant genes. Front Bioscience-Landmark 14(12):4457–4463
Gianluigi TAVERNA et al (2015) Inflammation and prostate cancer: friends or foe? Inflamm Res v 64:275–286
Gingrich JR et al (1999) Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prost Cancer Prost Dis 2(2):70–75
Hermes TA et al (2019) Tempol treatment shows phenotype improvement in mdx mice. PLoS One 14(4):e0215590
Hochberg DA (2002) Decreased suburethral prostatic microvessel density in finasteride treated prostates: a possible mechanism for reduced bleeding in benign prostatic hyperplasia. J Urol (4):1731–1733
Huang YJ et al (2019) Oxidative stress-induced angiogenesis. J Clin Neurosci 63:13–16
Huss WJ et al (2001) Angiogenesis and prostate cancer: identification of a molecular progression switch. Cancer Res 61(6):2736–2743
INCA (2023) Câncer de Próstata. Disponível . https://www.inca.gov.br/tipos-de-cancer/cancer-de-prostata. Access: May 14th
Isabela ROSSETTO et al (2022) Tempol differential effect on prostate cancer inflammation: In vitro and in vivo evaluation. The Prostate 83(5):403–415. https://doi.org/10.1002/pros.24473
Jerrold ZAR (1999) H. Biostatistical analysis. Pearson Education India
Junqueira LC, Carneiro J (1979) Histoquímica e Citoquímica. Histologia Básica.
Labanca E et al (2015) Association of HO-1 and BRCA1 Is critical for the maintenance of cellular homeostasis in prostate cancer BRCA1 activates HO-1 transcription. Mol Cancer Res 13(11):1455–1464
Le Guelte, A et al (2011) Jumping the barrier: VE-cadherin, VEGF and other angiogenic modifiers in cancer. Biol Cell 103(12):593–605
Malik SS et al (2018) Risk factors for prostate cancer: a multifactorial case-control study. Curr Probl Cancer 42(3):337–343
Marzioni D et al (2023) NRF2 modulation in TRAMP mice: an in vivo model of prostate cancer. Mol Biology Rep 50(1):873–881
Melegh Z et al (2019) Targeting angiogenesis in prostate cancer. Int J Mol Sci 20(11):2676
Montico F et al (2013) Angiogenic and tissue remodeling factors in the prostate of elderly rats submitted to hormonal replacement. The Anat Rec 296(11):1758–1767
Montico F et al (2015) Reactive stroma in the prostate during late life: the role of microvasculature and antiangiogenic therapy influences The Prostate 75(14):1643–1661
Nishida N et al (2006) Angiogenesis in cancer. Vasc Health Risk Manag 2(3):213–219
Niu YN et al (2009) Stroma–epithelium crosstalk in prostate cancer. Asian J Androl 11(1):28
Muscoli C et al (2003) On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol 140(3):445–460
Ohtake S et al (2018) Oxidative stress marker 8-hydroxyguanosine is more highly expressed in prostate cancer than in benign prostatic hyperplasia. Mol Clin Oncol 9(3):302–304
Olimi AF (2000) Carcinoma-associated fibroblasts stimulate tumor progression of initiated human epithelium. Breast Cancer Res v 2:1–1
Rebello R (2021) Prostate cancer. Nature reviews. Dis Prim. https://doi.org/10.1038/s41572-020-00243-0
Reuter S et al (2010) Oxidative stress, inflammation, and cancer: how are they linked ? Free Radic Biol Med 49(11):1603–1616
Rizzi E et al (2013) Tempol inhibits TGF-β and MMPs upregulation and prevents cardiac hypertensive changes. Int J Cardiol (1):165–173
Rowley DR (1998) What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev 17:411–419
Sciarra A et al (2008) Prostate growth and inflammation. J Steroid Biochem Mol Biol 108:3–5
Sfanos KS et al (2018) The inflammatory microenvironment and microbiome in prostate cancer development. Nat Reviews Urol 15(1):11–24
Shashni B et al (2021) Management of tumor growth and angiogenesis in triple-negative breast cancer by using redox nanoparticles. Biomaterials 269:120645
SHULIN ARUNS, Li (2011) Vimentin as a potential molecular target in cancer therapy or vimentin, an overview and its potential as a molecular target for cancer therapy. Cell Mol Life Sci v 68:3033–3046
Siegel RL et al (2023) Cancer statistics. CA: a cancer journal for clinicians 68(1):7–30
Silva HNM et al (2021) Oxidative stress, inflammation, and activators of mitochondrial biogenesis: Tempol targets in the diaphragm muscle of exercise trained-mdx mice. Front Physiol 12:649793
Thomas R et al (2012) A novel class of androgen receptor inhibitors that suppresses castration-resistant growth of prostate cancer SOD Mimetics: a novel class of AR inhibitors. Mol Cancer Ther 11(1):87–97
Tomas D et al (2010) Intensity of stromal changes predicts biochemical recurrence-free survival in prostatic carcinoma. Scand J Urol Nephrol 44(5):284–290
Tuxhorn JA (2002a) Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 8:2912–2923
Tuxhorn JA et al (2002b) Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. , v. 62, n. 11. Cancer Res 62(11):3298–3307
Tuxhornja JA, Gustavo E et al (2001) Reactive stroma in prostate cancer progression. J Urol. https://doi.org/10.1016/S0022-5347(05)65620-0
Van Moorselaar RJA et al (2002) Angiogenesis in prostate cancer: its role in disease progression and possible therapeutic approaches. Mol Cell Endocrinol. https://doi.org/10.1016/S0303-7207(02)00262-9
Verona EV et al (2007) Transforming growth factor-β signaling in prostate stromal cells supports prostate carcinoma growth by up-regulating stromal genes related to tissue remodeling. Cancer Research 67(12):5737–5746
Vestweber D (2008) VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol 28(2):223–232
Wang X et al (2020) Molecular bases of VEGFR-2-mediated physiological function and pathological role. Front Cell Dev Biol 8:599281
Weidner N et al (1993) Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. The Am J pathol 143(2):401
Wilcox CS (2010) Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther 126(2):119–145
Xi L et al (2023) New advances in the research of clinical treatment and novel anticancer agents in tumor angiogenesis. Biomed Pharmacother 163:114806
Yeo C et al (2019) Serum promotes vasculogenic mimicry through the EphA2/VE-cadherin/AKT pathway in PC-3 human prostate cancer cells. Life Sci 221:267–273
Acknowledgements
Thanks the Multidisciplinary Center for Biological Investigation on Laboratory Animal Science (CEMIB)/UNICAMP.
Funding
This study was supported by the Coordination for the improvement of higher Education Personnel – Brasil (CAPES) – Finance Code 001 and São Paulo Research Foundation – FAPESP (2018/21647-6 and 2021/02108-0).
Author information
Authors and Affiliations
Contributions
CVHA and SFR: These authors proposed the main manuscript idea and wrote the manuscript text. And also developed the experiment. RIMU: She also took part the experimental tecnics. SFR: He prepared figures. All authors reviewed the manuscript and wrote some part of the text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, F.R., Rossetto, I.M.U., Montico, F. et al. Differential tempol effects in prostatic cancer: angiogenesis and short- and long-term treatments. J Mol Histol (2024). https://doi.org/10.1007/s10735-024-10187-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10735-024-10187-4