Abstract
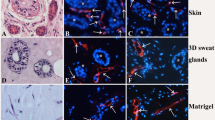
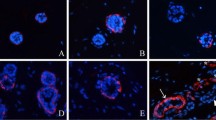
Epidermal basal cells invaginate into the dermis to form sweat ducts, which then grow downwards further to form secretory coils during the ontogenesis of eccrine sweat glands, but the time course of differentiation of different cell types in 3D-reconstructed eccrine sweat glands remain unclear. In this study, secretory cell-specific marker K7, clear secretory cell-specific marker CA II, dark secretory cell-specific marker GCDFP-15, myoepithelial cell-specific marker α-SMA, inner duct cell-specific marker S100P and outer duct cell-specific marker S100A2 were detected by immunofluorescence staining. The results showed that S100P and S100A2 were first detected at 2 weeks post implantation, K7 and α-SMA at 3 weeks, and GCDFP-15 and CA II at 4 weeks. The differentiation of ducts preceded secretory coils in 3D-reconstructed eccrine sweat glands. After 8 weeks post implantation, the distribution of these markers in 3D-reconstructed eccrine sweat glands was similar to that in native ones, and the percentage of the 3D-reconstructed glands expressing these markers maintained steady. We conclude that although the 3D-reconstructed and native eccrine sweat glands originated from different cells, the differentiation of different cell types in 3D-reconstructed eccrine sweat glands parallels the sequence observed during embryonic development.







Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- α-SMA:
-
α-Smooth muscle actin
- CA II:
-
Carbonic anhydrase II
- CEA:
-
Carcinoembryonic antigen
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- FoxA1:
-
Forkhead transcription factor A1
- GCDFP-15:
-
Gross cystic disease fluid protein-15
- HE:
-
Hematoxylin–eosin
- NKCC1:
-
Na+–K+–2Cl-cotransporter 1
- PBS:
-
Phosphate-buffered saline
- SUMC:
-
Shantou University Medical College
References
Boni R, Burg G, Doguoglu A, Ilg EC, Schafer BW, Muller B, Heizmann CW (1997) Immunohistochemical localization of the Ca2+ binding S100 proteins in normal human skin and melanocytic lesions. Br J Dermatol 137:39–43
Bovell DL, MacDonald A, Meyer BA, Corbett AD, MacLaren WM, Holmes SL, Harker M (2011) The secretory clear cell of the eccrine sweat gland as the probable source of excess sweat production in hyperhidrosis. Exp Dermatol 20:1017–1020
Cheshire WP, Freeman R (2003) Disorders of sweating. Semin Neurol 23:399–406
Cui CY, Schlessinger D (2015) Eccrine sweat gland development and sweat secretion. Exp Dermatol 24:644–650
Cui CY, Childress V, Piao Y, Michel M, Johnson AA, Kunisada M, Ko MS, Kaestner KH, Marmorstein AD, Schlessinger D (2012) Forkhead transcription factor FoxA1 regulates sweat secretion through Bestrophin 2 anion channel and Na-K-Cl cotransporter 1. Proc Natl Acad Sci USA 109:1199–1203
Fu X, Fang L, Li X, Cheng B, Sheng Z (2006) Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen 14:325–335
Heller DS, Haefner HK, Hameed M, Lieberman RW (2002) Vulvar hidradenitis suppurativa. Immunohistochemical evaluation of apocrine and eccrine involvement. J Reprod Med 47:695–700
Li H, Chen L, Zeng S, Li X, Zhang X, Lin C, Zhang M, Xie S, He Y, Shu S, Yang L, Tang S, Fu X (2015a) Matrigel basement membrane matrix induces eccrine sweat gland cells to reconstitute sweat gland-like structures in nude mice. Exp Cell Res 332:67–77
Li H, Li X, Zhang M, Chen L, Zhang B, Tang S, Fu X (2015b) Three-dimensional co-culture of BM-MSCs and eccrine sweat gland cells in Matrigel promotes transdifferentiation of BM-MSCs. J Mol Histol 46:431–438
Li H, Li X, Zhang B, Zhang M, Chen W, Tang S, Fu X (2016) Changes in keratins and alpha-smooth muscle actin during three-dimensional reconstitution of eccrine sweat glands. Cell Tissue Res 365:113–122
Li H, Chen L, Zhang M, Zhang B (2017a) Cells in 3D-reconstitutued eccrine sweat gland cell spheroids differentiate into gross cystic disease fluid protein 15-expressing dark secretory cells and carbonic anhydrase II-expressing clear secretory cells. Acta Histochem 119:620–623
Li H, Chen L, Zhang M, Zhang B (2017b) Foxa1 gene and protein in developing rat eccrine sweat glands. J Mol Histol 48:1–7
Li H, Zhang M, Chen L, Zhang B, Zhang C (2017c) Expression of S100A2 and S100P in human eccrine sweat glands and their application in differentiating secretory coil-like from duct-like structures in the 3D reconstituted eccrine sweat spheroids. J Mol Histol 48:219–223
Li X, Li H, Zhang M, Chen L, Zhang B (2017d) Cell proliferation and differentiation during the three dimensional reconstitution of eccrine sweat glands. J Mol Histol 48:113–120
Lu C, Fuchs E (2014) Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb Perspect Med 4:a015222
Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N, Blanpain C, Fuchs E (2012) Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 150:136–150
Moll I, Moll R (1992) Changes of expression of intermediate filament proteins during ontogenesis of eccrine sweat glands. J Invest Dermatol 98:777–785
Moll R, Moll I, Wiest W (1982) Changes in the pattern of cytokeratin polypeptides in epidermis and hair follicles during skin development in human fetuses. Differentiation 23:170–178
Nagamoto T, Eguchi G, Beebe DC (2000) Alpha-smooth muscle actin expression in cultured lens epithelial cells. Invest Ophthalmol Vis Sci 41:1122–1129
Saga K (2002) Structure and function of human sweat glands studied with histochemistry and cytochemistry. Prog Histochem Cytochem 37:323–386
Sato K, Kang WH, Saga K, Sato KT (1989) Biology of sweat glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol 20:537–563
Schon M, Benwood J, O’Connell-Willstaedt T, Rheinwald JG (1999) Human sweat gland myoepithelial cells express a unique set of cytokeratins and reveal the potential for alternative epithelial and mesenchymal differentiation states in culture. J Cell Sci 112(Pt 12):1925–1936
Shrestha P, Muramatsu Y, Kudeken W, Mori M, Takai Y, Ilg EC, Schafer BW, Heizmann CW (1998) Localization of Ca2+-binding S100 proteins in epithelial tumours of the skin. Virchows Arch 432:53–59
Van Muijen GN, Warnaar SO, Ponec M (1987) Differentiation-related changes of cytokeratin expression in cultured keratinocytes and in fetal, newborn, and adult epidermis. Exp Cell Res 171:331–345
Zhu L, Okano S, Takahara M, Chiba T, Tu Y, Oda Y, Furue M (2013) Expression of S100 protein family members in normal skin and sweat gland tumors. J Dermatol Sci 70:211–219
Acknowledgements
The manuscript was supported in part by the National Natural Science Foundation of China (81772102, 81471882).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare there is no conflict of interest and no competing financial, personal or other relationships with other people or organizations.
Rights and permissions
About this article
Cite this article
Zhang, M., Li, H., Xie, S. et al. Time course of differentiation of different cell types in 3D-reconstructed eccrine sweat glands. J Mol Hist 49, 567–575 (2018). https://doi.org/10.1007/s10735-018-9795-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-018-9795-y