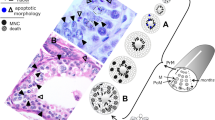
Abstract
In a previous study on the effects of gestational and lactational exposure of para-nonylphenol on male rats, we noted in both induced and uninduced rats, that variations in cleaved caspase-3 immunostaining patterns were associated with distinct nuclear alterations in mainly basally located germ cells (spermatogonia and preleptotene spermatocytes). These were re-analysed and compared with cleaved caspase-3-labeled germ cells in the aging human and the spermatogenically active catfish testis. In the rat testes, cytoplasmic immunostaining was progressively associated with lateral compression of the nucleus, its break up into large pieces which can contain immunostained marginated chromatin masses. The pale remnants of the nucleus continued to shrink in size concomitant with the appearance of blue-purplish stained regions in the cytoplasm similar in color to the condensed chromatin in spermatids, a condition which was TUNEL-negative. These large clumps of chromatin also eventually disappeared, giving rise to cells resembling cytoplasmic ghosts, a condition which was TUNEL-positive. By contrast, the immunolabeled nuclei of human and catfish germ cells condensed into a single mass, after which they lost immunoreactivity. To exclude the possibility that these observations could reflect alterations in Sertoli nuclei, rat testicular sections were probed with a mouse anti-human GATA-4 monoclonal (MHM) antibody. The MHM was, however, the second of two GATA-4 antibodies tested, with a goat anti-mouse polyclonal (GMP) initially used to label the rat Sertoli nuclei. GMP unexpectedly, but distinctly labeled the complete development of the acrosome in the rat testis, a fortuitous finding with utility for staging of the seminiferous epithelium.





Similar content being viewed by others
References
Allan DJ, Harmon BV, Kerr JFR (1987) Cell death in spermatogenesis. In: Potten CS (ed) Perspectives on mammalian cell death. Oxford University Press, London, pp 229–258
Allan DJ, Harmon BV, Roberts SA (1992) Spermatogonial apoptosis has three morphologically recognizable phases and shows no circadian rhythm during normal spermatogenesis in the rat. Cell Prolif 25:241–250
Baba S, Heike T, Umeda K, Iwasa T, Kaichi S, Hiraumi Y, Doi H, Yoshimoto M, Kanatsu-Shinohara M, Shinohara T, Nakahata T (2007) Generation of cardiac and endothelial cells from neonatal mouse testis-derived multipotent germline stem cells. Stem Cells 25:1375–1383
Bartke A (1995) Editorial: apoptosis of male germ cells, a generalized or a cell type-specific phenomenon? Endocrinology 136:3–4
Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh AJW (1995) Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology 136:5–12
Borisov AB, Carlson BM (2000) Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Record 258:305–318
Burgoyne LA (1999) The mechanisms of pyknosis: hypercondensation and death. Exp Cell Res 248:214–222
Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP (1998) Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci 18:4914–4928
Clarke PGH (1990) Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol 181:195–213
Coers W, Timens W, Kempinga C, Klok PA, Moshage H (1998) Specificity of antibodies to nitric oxide synthase isoforms in human, guinea pig, rat, and mouse tissues. J Histochem Cytochem 46:1385–1391
Collins JA, Schandl CA, Young KK, Vesely J, Willingham MC (1997) Major DNA fragmentation is a late event in apoptosis. J Histochem Cytochem 45:923–934
de Jager C, Bornman MS, Oosthuizen MC (1999) II. The effect of p-nonylphenol on the fertility potential of male rats after gestational, lactational and direct exposure. Andrologia 31:107–113
De Rooij DG, Russell LD (2000) All you wanted to know about spermatogonia but were afraid to ask. J Androl 21:776–798
Devine PJ, Payne CM, McCuskey MK, Hoyer PB (2000) Ultrastructural evaluation of oocytes during atresia in rat ovarian follicles. Biol Reprod 63:1245–1252
Dini L, Coppola S, Ruzittu MT, Ghibelli L (1996) Multiple pathways for apoptotic nuclear fragmentation. Exp Cell Res 223:340–347
Feranil JB, Isobe N, Nakao T (2005) Apoptosis in the antral follicles of swamp buffalo and cattle ovary: TUNEL and caspase-3 histochemistry. Reprod Domest Anim 40:111–116
Gerner C, Gotzmann J, Fröhwein U, Schamberger C, Ellinger A, Sauermann G (2002) Proteome analysis of nuclear matrix proteins during apoptotic chromatin condensation. Cell Death Differ 9:671–681
Ghosh S, Sinha-Hikim AP, Russell LD (1991) Further observations of stage-specific effects seen after short-term hypophysectomy in the rat. Tissue Cell 23:613–630
Huang Y, Lu K (2001) TUNEL staining and electron microscopy studies of apoptotic changes in the guinea pig vallate taste cells after unilateral glossopharyngeal denervation. Anat Embryol 204:493–501
Huckins C (1978) The morphology and kinetics of spermatogonial degeneration in normal adult rats: an analysis using a simplified classification of the germinal epithelium. Anat Record 190:905–926
Hurst PR, Mora JM, Fenwick MA (2006) Caspase-3, TUNEL and ultrastructural studies of small follicles in adult human ovarian biopsies. Hum Reprod 21:1974–1980
Jahnukainen K, Chrysis D, Hou M, Parvinen M, Eksborg S, Soder O (2004) Increased apoptosis occurring during the first wave of spermatogenesis is stage-specific and primarily affects mid-pachytene spermatocytes in the rat testis. Biol Reprod 70:290–296
Johnson L, Staub C, Neaves WB, Yanagimachi R (2001) Live human germ cells in the context of their spermatogenic stages. Hum Reprod 16:1575–1582
Kamada S, Kikkawa U, Tsjujimoto Y, Hunter T (2005) Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s). J Biol Chem 280:857–860
Ketola I, Pentikäinen V, Vaskivuo T, Ilvesmäki V, Herva R, Dunkel L, Tapanainen JS, Toppari J, Heikinheimo M (2000) Expression of transcription factor GATA-4 during human testicular development and disease. J Clin Endocrinol Metab 85:3925–3931
Kierszenbaum AL, Tres LL (2004) The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol 67:271–284
Kihlmark M, Rustum C, Eriksson C, Beckman M, Iverfeldt K, Hallberg E (2004) Correlation between nucleocytoplasmic transport and caspase-3-dependent dismantling of nuclear pores during apoptosis. Exp Cell Res 293:346–356
Malmi R, Söderström KO (1988) Lectin binding to rat spermatogenic cells: effects of different fixation methods and proteolytic enzyme treatment. Histochem J 20:276–282
Marshall GR, Ramaswamy S, Plant TM (2005) Gonadotropin-independent proliferation of the pale type A spermatogonia in the adult rhesus monkey (Macaca mulatta). Biol Reprod 73:222–229
McClusky LM (2005) Stage and season effects on cell cycle and apoptotic activities of germ cells and Sertoli cells during spermatogenesis in the spiny dogfish (Squalus acanthias). Reproduction 129:89–102
McClusky LM (2006) Stage-dependency of apoptosis and the blood-testis barrier in the dogfish shark (Squalus acanthias): cadmium-induced changes as assessed by vital fluorescence techniques. Cell Tissue Res 325:541–553
McClusky LM, De Jager C, Bornman MS (2007) Stage-related increase in the proportion of apoptotic germ cells and altered frequencies of stages in the spermatogenic cycle following gestational, lactational and direct exposure of male rats to p-nonylphenol. Toxicol Sci 95:249–256
McClusky LM, Barnhoorn IEJ, Van Dyk JC, Bornman MS (2008) Testicular apoptosis in feral Clarias gariepinus using TUNEL and cleaved caspase-3 immunohistochemistry. Ecotoxicol Environ Saf 71:41–46
McCoard SA, Lunstra DD, Wise TH, Ford JJ (2001) Specific staining of Sertoli cell nuclei and evaluation of Sertoli cell number and proliferative activity in Meishan and White Composite Boars during the neonatal period. Biol Reprod 64:689–695
Moreno RD, Lizama C, Urzúa N, Vergara SP, Reyes JG (2006) Caspase activation throughout the first wave of spermatogenesis in the rat. Cell Tissue Res 325:533–540
Nistal M, Codesal J, Paniagua R, Sanatmaria L (1987) Decrease in the number of human Ap and Ad spermatogonia and in the Ap/Ad ratio with advancing age. New data of the spermatogonial stem cell. J Androl 8:64–68
Ortiz R, Echeverria OM, Salgado R, Escobar ML, Vázquez-Nin GH (2006) Fine structural and cytochemical analysis of the processes of cell death of oocytes in atretic follicles in new born and prepubertal rats. Apoptosis 11:25–37
Park MS, Park P, Takeda M (2009) Starvation induces apoptosis in the midgut nidi of Periplaneta americana: a histochemical and ultrastructural study. Cell Tissue Res 335:631–638
Ramuz O, Isnardon D, Devilard E, Charafe-Jauffret E, Hassoun J, Birg F, Xerri L (2003) Constitutive nuclear localization and initial cytoplasmic apoptotic activation of endogenous caspase-3 evidenced by confocal microscopy. Int J Exp Pathol 84:75–81
Robertson JD, Orrenius S, Zhivotovsky B (2000) Review: nuclear events in apoptosis. J Struct Biol 129:346–358
Russell LD, Clermont Y (1977) Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat Record 187:347–366
Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED (1990) Histological and histopathological evaluation of the testis. Cache River Press, Clearwater, FL
Russell LD, Chiarini-Garcia H, Korsmeyer SJ, Knudson CM (2002) Bax-dependent spermatogonia apoptosis is required for testicular development and spermatogenesis. Biol Reprod 66:950–958
Sakamoto MK, Mima S, Kihara T, Tanimura T (2004) Sequential morphological changes of erythrocyte apoptosis in Xenopus larvae exposed to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCCD). Anat Record 279A:652–663
Sawhney P, Giammona J, Meistrich ML, Richburg JH (2005) Cisplatin-induced long-term failure of spermatogenesis in adult C57/B1/6J mice. J Androl 26:136–145
Sharpe RM, Maddocks S, Kerr JB (1990) Cell-cell interactions in the control of spermatogenesis as studied using Leydig cell destruction and testosterone replacement. Am J Anat 188:3–20
Sinha Hikim AP, Swerdloff RS (1999) Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod 4:38–47
Sinha Hikim AP, Rajavashisth TB, Sinha Hikim I, Lue Y, Bonavera JJ, Leung A, Wang C, Swerdloff RS (1997) Significance of apoptosis in the temporal and stage-specific loss of germ cells in the adult rat after gonadotropin deprivation. Biol Reprod 57:1193–1201
Stadelmann C, Lassmann H (2000) Detection of apoptosis in tissue sections. Cell Tissue Res 301:19–31
Suciu D (1983) Cellular death by apoptosis in some radiosensitive and radioresistant mammalian tissues. J Theor Biol 105:391–401
Toshimori K, Ito C (2003) Formation and organization of the mammalian sperm head. Arch Histol Cytol 66:383–396
Tsutsumi Y, Kamoshida S (2003) Pitfalls and caveats in histochemically demonstrating apoptosis. Acta Histochem Cytochem 36:271–280
Viger RS, Mertineit C, Trasler JM, Nemer M (1998) Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance promoter. Development 125:2665–2675
Wang R, Nakane PK, Koji T (1998) Autonomous cell death of male germ cells during fetal and postnatal period. Biol Reprod 58:1250–1256
Acknowledgments
The authors express their appreciation to the Department of Physiology, University of Pretoria for laboratory facilities, Prof. J.H.J van Vuren of the Department Zoology, University of Johannesburg for providing laboratory aquarium facilities for the rearing of the catfish, and Dr EH Abdel Goad of the Department of Urology, Nelson Mandela School of Medicine, University of KwaZulu Natal for supplying the human testicular tissues. Financial support from the National Research Foundation of South Africa (Project no’s. NRF 9657, NRF 9657) and the Water Research Commission of South Africa (K5/1505) is acknowledged. The authors thank Mrs. Joey Breedt of the Department of Veterinary Pathology, Faculty of Veterinary Sciences, University of Pretoria for tissue sectioning, Mr. Alan Hall of the Laboratory for Microscopy and Microanalysis, University of Pretoria for assistance with digital photomicrography, and Dr. Ross Wakelin for his comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McClusky, L.M., Patrick, S., Barnhoorn, I.E.J. et al. Immunohistochemical study of nuclear changes associated with male germ cell death and spermiogenesis. J Mol Hist 40, 287–299 (2009). https://doi.org/10.1007/s10735-009-9240-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-009-9240-3