Abstract
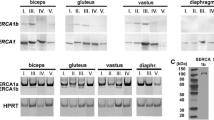
The examination was performed whether aquaporin (AQP) 9 is expressed in normal skeletal muscle at mRNA and protein levels. Gel electrophoresis of the reverse transcription-polymerase chain reaction (RT-PCR) product of total RNA samples of human normal muscles by oligonucleotide primers for human AQP9 showed a band of 221 basepairs, which corresponded to the basepair length between two primers of AQP9. The nucleotide sequence of RT-PCR product coincided with that of human AQP9. Immunoblot analysis revealed that the rabbit and sheep antibodies against the synthetic peptide of the C-terminal cytoplasmic domain of human AQP9 molecule reacted with a protein of approximately 30 kDa molecular weight in extracts of human normal skeletal muscles. Immunohistochemistry with our anti-AQP9 antibodies showed an immunoreaction at the myofiber surface of both type 1 and type 2 fibers with almost equal staining intensity in human skeletal muscles. The implication of AQP9 expression in skeletal myofibers was discussed.




Similar content being viewed by others
References
Ahrén B, Lundquist I (1981) Insulin secretory response to different secretagogues in hyper- and hypothyroid mice. Acta Endocrinol (Copenh) 97:508–513
Baba H, Zhang XJ, Wolfe RR (1995) Glycerol gluconeogenesis in fasting humans. Nutrition 11:149–153
Badaut J, Regli L (2004) Distribution and possible roles of aquaporin 9 in the brain. Neuroscience 129:971–981. doi:10.1016/j.neuroscience.2004.06.035
Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S, Agre P (2003) Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci USA 100:2945–2950. doi:10.1073/pnas.0437994100
Coppack SW, Jensen MD, Miles JM (1994) In vivo regulation of lipolysis in humans. J Lipid Res 35:177–193
Czech MP, Malbon CC, Kerman K, Gitomer W, Pilch PF (1980) Effect of thyroid status on insulin action in rat adipocytes and skeletal muscle. J Clin Invest 66:574–582. doi:10.1172/JCI109889
Elkjaer M, Vajda Z, Nejsum LN, Kwon T, Jensen UB, Amiry-Moghaddam M, Frøkiaer J, Nielsen S (2000) Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Commun 276:1118–1128. doi:10.1006/bbrc.2000.3505
Germani D, Puglianiello A, Cianfarani S (2008) Uteroplacental insufficiency down regulates insulin receptor and affects expression of key enzymes of long-chain fatty acid (LCFA) metabolism in skeletal muscle at birth. Cardiovasc Diabetol 7:14. doi:10.1186/1475-2840-7-14
Kabadi UM, Eisenstein AB (1980) Glucose intolerance in hyperthyroidism: role of glucagon. J Clin Endocrinol Metab 50:392–396
Kishida K, Kuriyama H, Funahashi T, Shimomura I, Kihara S, Ouchi N, Nishida M, Nishizawa H, Matsuda M, Takahashi M, Hotta K, Nakamura T, Yamashita S, Tochino Y, Matsuzawa Y (2000) Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem 275:20896–20902. doi:10.1074/jbc.M001119200
Kuriyama H, Shimomura I, Kishida K, Kondo H, Furuyama N, Nishizawa H, Maeda N, Matsuda M, Nagaretani H, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y (2002) Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes 51:2915–2921
Newsholme EA, Taylor K (1969) Glycerol kinase activities in muscles from vertebrates and invertebrates. Biochem J 112:465–474
Nicchia GP, Frigeri A, Nico B, Ribatti D, Svelto M (2001) Tissue distribution and membrane localization of aquaporin-9 water channel: evidence for sex-linked differences in liver. J Histochem Cytochem 49:1547–1556
O’Brien RM, Granner DK (1996) Regulation of gene expression by insulin. Physiol Rev 76:1109–1161
O’Brien RM, Streeper RS, Ayala JE, Stadelmaier BT, Hornbuckle LA (2001) Insulin-regulated gene expression. Biochem Soc Trans 29:552–558
Ontko JA (1986) Lipid metabolism in muscle. In: Engel AG, Banker BQ (eds) Myology. McGraw-Hill, New York, pp 697–720
Pastor-Soler N, Isnard-Bagnis C, Herak-Kramberger C, Sabolic I, Van Hoek A, Brown D, Breton S (2002) Expression of aquaporin 9 in the adult rat epididymal epithelium is modulated by androgens. Biol Reprod 66:1716–1722
Previs SF, Brunengraber H (1998) Methods for measuring gluconeogenesis in vivo. Curr Opin Clin Nutr Metab Care 1:461–465
Rizza RA, Mandarino LJ, Gerich JE (1982) Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab 54:131–138
Rosenfeld RG, Wilson DM, Dollar LA, Bennett A, Hintz RL (1982) Both human pituitary growth hormone and recombinant DNA-derived human growth hormone cause insulin resistance at a postreceptor site. J Clin Endocrinol Metab 54:1033–1038
Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA (1999) Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am J Physiol 277:F685–F696
Wakayama Y, Jimi T, Takeda A, Misugi N, Kumagai T, Miyake S, Shibuya S (1990) Immunoreactivity of antibodies raised against synthetic peptide fragments predicted from mid portions of dystrophin cDNA. J Neurol Sci 97:241–250
Wakayama Y, Jimi T, Inoue M, Kojima H, Shibuya S, Murahashi M, Hara H, Oniki H (2002) Expression of aquaporin 3 and its localization in normal skeletal myofibers. Histochem J 34:331–337
Wakayama Y, Inoue M, Kojima H, Jimi T, Shibuya S, Hara H, Oniki H (2004) Expression and localization of aquaporin 7 in normal skeletal myofiber. Cell Tissue Res 316:123–129. doi:10.1007/s00441-004-0857-y
Wang W, Hart PS, Piesco NP, Lu X, Gorry MC, Hart TC (2003) Aquaporin expression in developing human teeth and selected orofacial tissues. Calcif Tissue Int 72:222–227. doi:10.1007/s00223-002-1014-9
Yang B, Verbavatz JM, Song Y, Vetrivel L, Manley G, Kao WM, Ma T, Verkman AS (2000) Skeletal muscle function and water permeability in aquaporin-4 deficient mice. Am J Physiol Cell Physiol 278:C1108–C1115
Acknowledgments
The authors would like to thank Mrs. T. Nagami for her typing of this manuscript. Thanks were also extended to Mrs. Y. Hirayama for her technical help. This work was partly supported by the Research Grant (17A-10 and 20B-13) for Nervous and Mental Disorders from the Ministry of Health, Labour and Welfare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inoue, M., Wakayama, Y., Kojima, H. et al. Aquaporin 9 expression and its localization in normal skeletal myofiber. J Mol Hist 40, 165–170 (2009). https://doi.org/10.1007/s10735-009-9226-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-009-9226-1