Summary
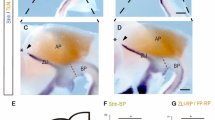
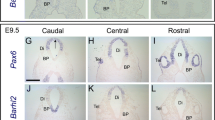
The Dickkopf (Dkk) family and Mmp9 are important for apoptosis and a number of other developmental processes. However, little is known about their roles in the development of cranial base, which is an important structure for coordinated development and growth of the craniofacial skeletons. In order to establish whether and in what way these genes are involved in cranial base development, we examined their expression patterns and cell apoptosis. Dkk1 was first seen in the perichondral mesenchyme in restricted domains from E14, and later in the migrating mesenchymal cells within the cartilage. Thereafter, it was widespread throughout the bones of the cranial base. The expression was downregulated in postnatal stages. Dkk2 was localized in the perichondral mesenchyme outlining the anterior cranial base in embryogenesis. Dkk3 was mainly detected in the occipital–vertebral joint at E13 and E14. Mmp9 transcripts were clustered in the inner layer of perichondral mesenchyme, juxtaposed with the terminally differentiated hypertrophic chondrocytes from E14. Later Mmp9-expressing cells were found at the sites of chondrocyte apoptosis. This was particularly clear at the distal ends of the synchondroses. These data indicate that Mmp9 regulates skeletogenesis in cranial base in a manner that is largely similar to that of the appendicular skeletons. Expression of Dkks suggests other roles that remain to be defined.
Similar content being viewed by others
References
Andersen E, Sonnesen L, Kjaer MS, Fischer Hansen B, Kjaer I. (2000). The prenatal cranial base complex and hand in Turner syndrome. Eur J Orthod 22:185–194
Captier G, Leboucq N, Bigorre M, Canovas F, Bonnel F, Bonnafe A, Montoya P. (2003). Plagiocephaly: morphometry of skull base asymmetry. Surg Radiol Anat 25:226–233
Chen Y, Zhao X. (1998). Shaping limbs by apoptosis. J Exp Zool 282:691–702
Couly GF, Coltey PM, Le Douarin NM. (1993). The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 117:409–429
Day TF, Guo X, Garrett-Beal L, Yang Y (2005). Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8:739–750
Eames BF, Helms JA. (2004). Conserved molecular program regulating cranial and appendicular skeletogenesis. Dev Dyn 231:4–13
Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM (2000). Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol 151:879–889
Enlow DH, McNamara JA, Jr. (1973). The neurocranial basis for facial form and pattern. Angle Orthod 43:256–270
Fjeld K, Kettunen P, Furmanek T, Kvinnsland IH, Luukko K (2005). Dynamic expression of Wnt signaling-related Dickkopf1, −2, and −3 mRNAs in the developing mouse tooth. Dev Dyn 233:161–166
Ford EHR (1958). growth of human cranial base. Am J Orthod 44:498–506
Gakunga PT, Kuboki Y, Opperman LA (2000). Hyaluronan is essential for the expansion of the cranial base growth plates. J Craniofac Genet Dev Biol 20:53–63
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992). Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. (1998). Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391:357–362
Gregory CA, Singh H, Perry AS, Prockop DJ (2003). The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem 278:28067–28078
Grotewold L, Ruther U (2002a). Bmp, Fgf and Wnt signalling in programmed cell death and chondrogenesis during vertebrate limb development: the role of Dickkopf-1. Int J Dev Biol 46:943–947
Grotewold L, Ruther U (2002b). The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. Embo J 21:966–975
Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C (2005). Canonical Wnt/beta-Catenin Signaling Prevents Osteoblasts from Differentiating into Chondrocytes. Dev Cell 8:727–738
Hilloowala RA, Trent RB, Pifer RG (1998). Interrelationships of brain, cranial base and mandible. Cranio 16:267–274
Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F (2005). Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 132:49–60
Ishii-Suzuki M, Suda N, Yamazaki K, Kuroda T, Senior PV, Beck F, Hammond VE (1999). Differential responses to parathyroid hormone-related protein (PTHrP) deficiency in the various craniofacial cartilages. Anat Rec 255:452–457
Jeffery N (2002). A high-resolution MRI study of linear growth of the human fetal skull base. Neuroradiology 44:358–366
Jemtland R, Lee K, Segre GV (1998). Heterogeneity among cells that express osteoclast-associated genes in developing bone. Endocrinology 139:340–349
Kawano Y, Kypta R (2003). Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116:2627–2634
Kreiborg S, Jensen BL, Larsen P, Schleidt DT, Darvann T (1999). Anomalies of craniofacial skeleton and teeth in cleidocranial dysplasia. J Craniofac Genet Dev Biol 19:75–79
Kreiborg S, Marsh JL, Cohen MM, Jr., Liversage M, Pedersen H, Skovby F, Borgesen SE, Vannier MW (1993). Comparative three-dimensional analysis of CT-scans of the calvaria and cranial base in Apert and Crouzon syndromes. J Craniomaxillofac Surg 21:181–188
Lewis AB, Roche AF (1988). Late growth changes in the craniofacial skeleton. Angle Orthod 58:127–135
Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, Harris SE, Rowe DW, Ke HZ, Wu D. (2005a). Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet 37:945–952
Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, Harris SE, Rowe DW, Ke HZ, Wu D. (2005b). Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet 39:945–952
MacDonald BT, Adamska M, Meisler MH (2004). Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development 131:2543–2552
Midtbo M, Wisth PJ, Halse A (1996). Craniofacial morphology in young patients with Turner syndrome. Eur J Orthod 18:215–225
Molsted K, Kjaer I, Dahl E (1993). Spheno-occipital synchondrosis in three-month-old children with clefts of the lip and palate: a radiographic study. Cleft Palate Craniofac J 30:569–573
Monaghan AP, Kioschis P, Wu W, Zuniga A, Bock D, Poustka A, Delius H, Niehrs C (1999). Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech Dev 87:45–56
Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Belmonte JC, Westphal H (2001). Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1:423–434
Nie X (2005a). Cranial base in craniofacial development: Developmental features, influence on facial growth, anomaly, and molecular basis. Acta Odontol Scand 63:127–135
Nie X (2005b) Dkk1, −2, and −3 expression in mouse craniofacial development. J Mol Histol 36: 367–372. DOI 10.1007/s10735-005-9008-3
Nie X, Luukko K, Kvinnsland IH, Kettunen P (2005). Developmentally regulated expression of Shh and Ihh in the developing mouse cranial base: Comparison with Sox9 expression. Anat Rec A Discov Mol Cell Evol Biol 286:891–898
Quintanilla JS, Biedma BM, Rodriguez MQ, Mora MT, Cunqueiro MM, Pazos MA (2002). Cephalometrics in children with Down’s syndrome. Pediatr Radiol 32:635–643
Rice DP, Rice R, Thesleff I (2003). Fgfr mRNA isoforms in craniofacial bone development. Bone 33:14–27
Rosenberg P, Arlis HR, Haworth RD, Heier L, Hoffman L, LaTrenta G (1997). The role of the cranial base in facial growth: Experimental craniofacial synostosis in the rabbit. Plast Reconstr Surg 99:1396–1407
Shum L, Wang X, Kane AA, Nuckolls GH (2003). BMP4 promotes chondrocyte proliferation and hypertrophy in the endochondral cranial base. Int J Dev Biol 47:423–431
Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD, Jr. (2003). The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349:2483–2494
Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z (1998). MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93:411–422
Vu TH, Werb Z (2000). Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev 14:2123–2133
Acknowledgements
The work was carried out at the department of Biomedicine and financially supported by the Faculty of Dentistry, University of Bergen. We would like to thank Ms Kjellfrid Haukanes for her skilful technique assistant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nie, X., Luukko, K., Fjeld, K. et al. Developmental expression of Dkk1-3 and Mmp9 and apoptosis in cranial base of mice. J Mol Hist 36, 419–426 (2005). https://doi.org/10.1007/s10735-005-9014-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-005-9014-5