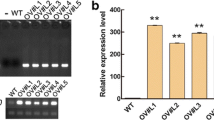
Abstract
Soybean is a valuable crop that provides protein and oil. The soybean yield and quality are affected by abiotic stresses viz. drought and heat stress. The process of poly(ADP-ribosyl)ation is an essential component of crucial cellular processes, including abiotic stress tolerance. This study is an attempt to unravel the function of Glycine max poly(ADP-ribose) polymerases (GmPARPs) using Agrobacterium-mediated transformation in soybean. The present study has identified and characterized the GmPARPs gene in soybean using bioinformatic and molecular analyses. Quantitative real-time PCR (qRT-PCR) results showed the differential expression of GmPARPs under drought and heat stress conditions. Further, to elucidate the function of the GmPARPs in drought and heat stress, soybean GmPARPs-RNAi transgenic lines were raised which were confirmed by molecular analyses. Various physiological traits such as Fv/Fm ratio, H2O2 production, and proline and chlorophyll content demonstrated the tolerance of GmPARPs-RNAi lines under drought and heat stress conditions. Besides, the role of stress-associated genes, Allene oxide synthase and Phenylalanine ammonia-lyase were studied to dissect the pathways involved in conferring drought and heat tolerance in GmPARPs-RNAi lines, which were consistent with the previous studies. Our study indicated that the downregulation of the GmPARP1 efficiently improves drought and heat tolerance in soybean. However, GmPARP2 knockdown could only protect soybean plants under drought stress. Thus, GmPARPs may also serve as an important target for the improvement of soybean cultivars and other crops.











Similar content being viewed by others
References
Aguilar-Quesada R, Muñoz-Gámez JA, Martín-Oliva D, Peralta A, Valenzuela MT, Matínez-Romero R, Quiles-Pérez R, Murcia JMD, de Murcia G, de Almodóvar MR, Oliver FJ (2007) Interaction between ATM and PARP-1 in response to DNA damage and sensitization of ATM deficient cells through PARP inhibition. BMC Mol Biol 8(1):1–8
Ahel I, Ahel D, Matsusaka T, Clark AJ, Pines J, Boulton SJ, West SC (2008) Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature 451:81–85
Ahel D, Hořejší Z, Wiechens N, Polo SE, Garcia-Wilson E, Ahel I, Flynn H, Skehel M, West SC, Jackson SP, Owen-Hughes T (2009) Poly (ADP-ribose)–dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 325(5945):1240–1243
Ahlfors R, Lång S, Overmyer K, Jaspers P, Broscheé M, Tauriainen A, Kollist H, Tuominen H, Belles-Boix E, Piippo M, Inzeé D (2004) Arabidopsis RADICAL-INDUCED CELL DEATH1 belongs to the WWE protein–protein interaction domain protein family and modulates abscisic acid, ethylene, and methyl jasmonate responses. Plant Cell 16(7):1925–1937
Alvarez-Gonzalez R, Mendoza-Alvarez H (1995) Dissection of ADP-ribose polymer synthesis into individual steps of initiation, elongation, and branching. Biochimie 77(6):403–407
Amor Y, Babiychuk E, Inze D, Levine A (1998) The involvement of poly(ADP-ribose) polymerase in the oxidative stress responses in plants. FEBS Lett 440:1–7
Aredia F, Scovassi AI (2014) Poly(ADP-ribose): a signaling molecule in different paradigms of cell death. Biochem Pharmacol 92:157–163
Armengaud P, Thiery L, Buhot N, Grenier-de March G, Savouré A (2004) Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant 120(3):442–450
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55(403):1607–1621
Betts SD, Basu S, Bolar J, Booth R, Chang S, Cigan AM, Farrell J, Gao H, Harkins K, Kinney A, Lenderts B (2019) Uniform expression and relatively small position effects characterize sister transformants in maize and soybean. Front Plant Sci 10:1209
Block MD, Verduyn C, Brouwer DD, Cornelissen M (2005) Poly (ADP-ribose) polymerase in plants affects energy homeostasis, cell death and stress tolerance. Plant J 41(1):95–106
Bologna NG, Voinnet O (2014) The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu Rev Plant Biol 65(1):473–503
Bonfiglio JJ, Fontana P, Zhang Q, Colby T, Gibbs-Seymour I, Atanassov I, Bartlett E, Zaja R, Ahel I, Matic I (2017) Serine ADP-ribosylation depends on HPF1. Mol Cell 65:932–940
Bouche N, Bouchez D (2001) Arabidopsis gene knockout: phenotypes wanted. Curr Opin Plant Biol 4(2):111–117
Brady PN, Goel A, Johnson MA (2019) Poly(ADP-ribose) polymerases in host-pathogen interactions, inflammation, and immunity. Microbiol Mol Biol Rev 83:e00038-e118
Brodersen P, Voinnet O (2006) The diversity of RNA silencing pathways in plants. Trends Genet 22(5):268–280
Chang P, Jacobson MK, Mitchison TJ (2004) Poly(ADP-ribose) is required for spindle assembly and structure. Nature 432:645–649
Chen H, Nelson RS, Sherwood JL (1994) Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection. Biotechniques 16(4):664–668
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ (2005) Real-time quantification of microRNAs by stem–loop RT–PCR. Nucleic Acids Res 33(20):179–179
Chetty VJ, García DJ, Narváez-Vásquez J, Orozco-Cárdenas ML (2020) Poly (ADP-ribose) polymerase inhibitor 3-methoxybenzamide enhances in vitro plant growth, microtuberization, and transformation efficiency of blue potato (Solanum tuberosum L. subsp. andigenum). In Vitro Cell Dev Biol-Plant 56:833–841
Coggill P, Finn RD, Bateman A (2008) Identifying protein domains with the Pfam database. Curr Protoc Bioinformatics Chapter 2: Unit 2.5
Cohen MS, Chang P (2018) Insights into the biogenesis, function, and regulation of ADP-ribosylation. Nat Chem Biol 14:236–243
Crawford K, Bonfiglio JJ, Mikoc A, Matic I, Ahel I (2018) Specificity of reversible ADP-ribosylation and regulation of cellular processes. Crit Rev Biochem Mol Biol 53:64–82
D’Amours D, Desnoyers S, D’Silva I, Poirier GG (1999) Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J 342:249–268
Dai S, Zheng P, Marmey P, Zhang S, Tian W, Chen S, Beachy RN, Fauquet C (2001) Comparative analysis of transgenic rice plants obtained by Agrobacterium-mediated transformation and particle bombardment. Mol Breed 7(1):25–33
Day CD, Lee E, Kobayashi J, Holappa LD, Albert H, Ow DW (2000) Transgene integration into the same chromosome location can produce alleles that express at a predictable level, or alleles that are differentially silenced. Genes Dev 14(22):2869–2880
de Murcia JM, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, Oliver FJ, Masson M, Dierich A, LeMeur M, Walztinger C, Chambon P, de Murcia G (1997) Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci USA 94:7303–7307
de Murcia JM, Ricoul M, Tartier L, Niedergang C, Huber A, Dantzer F, Schreiber V, Ame J-C, Dierich A, LeMeur M, Sabatier L, Chambon P, de Murcia G (2003) Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J 22:2255–2263
Demmig B, Björkman O (1987) Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 171(2):171–184
Desikan R, Reynolds A, Hancock TJ, Neill JS (1998) Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem J 330(1):115–120
Dong OX, Ronald PC (2021) Targeted DNA insertion in plants. PNAS 118(22):e2004834117
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Du H, Liu H, Xiong L (2013) Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front Plant Sci 4(397):1–10
Francastel C, Walters MC, Groudine M, Martin DI (1999) A functional enhancer suppresses silencing of a transgene and prevents its localization close to centromeric heterochromatin. Cell 99(3):259–269
Gagné JP, Isabelle M, Lo KS, Bourassa S, Hendzel MJ, Dawson VL, Dawson TM, Poirier GG (2008) Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes. Nucl Acid Res 36:6959–6976
Gakière B, Hao J, de Bont L, Pétriacq P, Nunes-Nesi A, Fernie AR (2018) NAD+ biosynthesis and signaling in plants. Crit Rev Plant Sci 37:259–307
Geissler T, Wessjohann LA (2011) A whole-plant microtiter plate assay for drought stress tolerance-inducing effects. J Plant Growth Regul 30(4):504–511
Gibson BA, Kraus WL (2012) New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol 13:411–424
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Gupte R, Liu ZY, Kraus WL (2017) PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes Dev 31:101–126
Haince JF, Kozlov S, Dawson VL, Dawson TM, Hendzel MJ, Lavin MF, Poirier GG (2007) Ataxia telangiectasia mutated (ATM) signaling network is modulated by a novel poly(ADP-ribose)-dependent pathway in the early response to DNA-damaging agents. J Biol Chem 282:16441–16453
Hayashi K, Tanaka M, Shimada T, Miwa M, Sugimura T (1983) Size and shape of poly(ADP-ribose): examination by gel filtration, gel electrophoresis and electron microscopy. Biochem Biophys Res Commun 112:102–107
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57(12):1332–1334
Hou L, Wang L, Wu X, Gao W, Zhang J, Huang C (2019) Expression patterns of two pal genes of Pleurotus ostreatus across developmental stages and under heat stress. BMC Microbiol 19:1–16
Hu T, Metz S, Chay C, Zhou HP, Biest N, Chen G, Cheng M, Feng X, Radionenko M, Lu F, Fry J (2003) Agrobacterium-mediated large-scale transformation of wheat (Triticum aestivum L.) using glyphosate selection. Plant Cell Rep 21(10):1010–1019
Huang J, Gu M, Lai Z, Fan B, Shi K, Zhou YH, Yu JQ, Chen Z (2010) Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol 153(4):1526–1538
Ishikawa K, Ogawa T, Hirosue E, Nakayama Y, Harada K, Fukusaki E, Yoshimura K, Shigeoka S (2009) Modulation of the poly(ADP-ribosyl)ation reaction via the Arabidopsis ADP-Ribose/NADH pyrophosphohydrolase, AtNUDX7, is involved in the response to oxidative stress. Plant Physiol 151:741–754
Ishizuka S, Martin K, Booth C, Potten CS, de Murcia G, Burkle A, Kirkwood TB (2003) Poly(ADP-ribose) polymerase-1 is a survival factor for radiation-exposed intestinal epithelial stem cells in vivo. Nucleic Acids Res 31:6198–6205
Jaspers P, Overmyer K, Wrzaczek M, Vainonen JP, Blomster T, Salojärvi J, Reddy RA, Kangasjärvi J (2010) The RST and PARP-like domain containing SRO protein family: analysis of protein structure, function and conservation in land plants. BMC Genom 11(1):1–20
Kanai M, Hanashiro K, Kim SH, Hanai S, Boulares AH, Miwa M, Fukasawa K (2007) Inhibition of Crm1-p53 interaction and nuclear export of p53 by poly(ADP-ribosyl)ation. Nat Cell Biol 9:1175–1183
Kang HC, Lee YI, Shin JH, Andrabi SA, Chi Z, Gagné JP, Lee Y, Ko HS, Lee BD, Poirier GG, Dawson VL (2011) Iduna is a poly (ADP-ribose)(PAR)-dependent E3 ubiquitin ligase that regulates DNA damage. PNAS 108(34):14103–14108
Kao WY, Forseth IN (1992) Diurnal leaf movement, chlorophyll fluorescence and carbon assimilation in soybean grown under different nitrogen and water availabilities. Plant Cell Environ 15:703–710
Kedar PS, Stefanick DF, Horton JK, Wilson SH (2008) Interaction between PARP-1 and ATR in mouse fibroblasts is blocked by PARP inhibition. DNA Rep 7:1787–1798
Kiehlbauch CC, Aboulela N, Jacobson EL, Ringer DP, Jacobson MK (1993) High-resolution fractionation and characterization of ADP-ribose polymers. Anal Biochem 208:26–34
Kohli A, Twyman RM, Abranches R, Wegel E, Stoger E, Christou P (2003) Transgene integration, organization and interaction in plants. Plant Mol Biol 52:247–258
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Biol 42(1):313–349
Kurata T, Yamamoto KT (1998) petit1, a conditional growth mutant of Arabidopsis defective in sucrose-dependent elongation growth. J Plant Physiol 118:793–801
Lamb RS, Citarelli M, Teotia S (2012) Functions of the poly (ADP-ribose) polymerase superfamily in plants. Cell Mol Life Sci 69(2):175–189
Lee GJ, Wu X, Shannon JG, Sleper DA, Nguyen HT (2007) Soybean. Oilseeds 2:1–53
Lepiniec L, Babiychuk E, Kushnir S, Van Montagu M, Inzé D (1995) Characterization of an Arabidopsis thaliana cDNA homologue to animal poly(ADP-ribose) polymerase. FEBS Lett 364:103–108
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79(4):583–593
Li XG, Chen SB, Lu ZX, Chang TJ, Zeng QC, Zhu Z (2002) Impact of copy number on transgene expression in tobacco. J Integr Plant Biol 44:120–123
Liu J, Zhu JK (1997) Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol 114:591–596
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Lloyd J, Meinke D (2012) A comprehensive dataset of genes with a loss-of-function mutant phenotype in Arabidopsis. Plant Physiol 158:1115–1129
Lorrain S, Lin B, Auriac MC, Kroj T, Saindrenan P, Nicole M, Balaguee C, Roby D (2004) Vascular associated death1, a novel gram domain-containing protein, is a regulator of cell death and defense responses in vascular tissues. Plant Cell 16(8):2217–2232
Lu C, Meyers BC, Green PJ (2007) Construction of small RNA cDNA libraries for deep sequencing. Methods 43(2):110–117
Margis R, Fusaro AF, Smith NA, Curtin SJ, Watson JM, Finnegan EJ, Waterhouse PM (2006) The evolution and diversification of Dicers in plants. FEBS Lett 580(10):2442–2450
Martín-Guerrero SM, Casado P, Hijazi M, Rajeeve V, Plaza-Díaz J, Abadía-Molina F, Navascués J, Cuadros MA, Cutillas PR, Martín-Oliva D (2020) PARP-1 activation after oxidative insult promotes energy stress-dependent phosphorylation of YAP1 and reduces cell viability. Biochem J 477(23):4491–4513
Matzke AJ, Matzke MA (1998) Position effects and epigenetic silencing of plant transgenes. Curr Opin Plant Biol 1:142–148
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence: a practical guide. J Exp Bot 51:659–668
Mendoza-Alvarez H, Alvarez-Gonzalez R (2001) Regulation of p53 sequence-specific DNA-binding by covalent poly(ADP-ribosyl)ation. J Biol Chem 276:36425–36430
Miller G, Suzuki N, Ciftci-Yilmazi N, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33:453–467
Min W, Bruhn C, Grigaravicius P, Zhou ZW, Li F, Krüger A, Siddeek B, Greulich KO, Popp O, Meisezahl C, Calkhoven CF (2013) Poly (ADP-ribose) binding to Chk1 at stalled replication forks is required for S-phase checkpoint activation. Nat Commun 4(1):1–14
Pathan MS, Lee JD, Shannon JG, Nguyen HT (2007) Recent advances in breeding for drought and salt stress tolerance in soybean. In: Jenks MA, Hasegawa PM, Jain SM (eds) Advances in molecular breeding toward drought and salt tolerant crops. Springer, Dordrecht, pp 739–773
Pérez-González A, Caro E (2019) Benefits of using genomic insulators flanking transgenes to increase expression and avoid positional effects. Sci Rep 9(1):1–11
Pikaart M, Feng J, Villeponteau B (1992) The polyomavirus enhancer activates chromatin accessibility on integration into the HPRT gene. Mol Cell Biol 12(12):5785–5792
Pikaart MJ, Recillas-Targa F, Felsenfeld G (1998) Loss of transcriptional activity of a transgene is accompanied by DNA methylation and histone deacetylation and is prevented by insulators. Genes Dev 12(18):2852–2862
Pleschke JM, Kleczkowska HE, Strohm M, Althaus FR (2000) Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J Biol Chem 275:40974–40980
Ramakrishnan M, Papolu PK, Satish L, Vinod KK, Wei Q, Sharma A, Emamverdian A, Zou LH, Zhou M (2022) Redox status of the plant cell determines epigenetic modifications under abiotic stress conditions and during developmental processes. J Adv Res 42:99–116
Reddy DS, Bhatnagar-Mathur P, Vadez V, Sharma KK (2012) Grain legumes (soybean, chickpea, and peanut): omics approaches to enhance abiotic stress tolerance. In: Tuteja N, Gill SS, Tiburcio AF, Tuteja R (eds) Improving crop resistance to abiotic stress, vol 1. Wiley, Hoboken, p 993
Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A (2004) Rational siRNA design for RNA interference. Nat Biotechnol 22:326–330
Rissel D, Heym PP, Thor K, Brandt W, Wessjohann LA, Peiter E (2017) No silver bullet - Canonical poly(ADP-ribose) polymerases (PARPs) are no universal factors of abiotic and biotic stress resistance of Arabidopsis thaliana. Front Plant Sci 8:59
Rizzo G, Baroni L (2018) Soy, soy foods and their role in vegetarian diets. Nutrients 10:43
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Schubert D, Lechtenberg B, Forsbach A, Gils M, Bahadur S, Schmidt R (2004) Silencing in Arabidopsis T-DNA transformants: the predominant role of a gene-specific RNA sensing mechanism versus position effects. Plant Cell 16(10):2561–2572
Schuhwerk H, Atteya R, Siniuk K, Wang ZQ (2017) PARPing for balance in the homeostasis of poly(ADP-ribosyl)ation. Semin Cell Dev Biol 63:81–91
Schulz P, Neukermans J, Van der Kelen K, Mühlenbock P, Van Breusegem F, Noctor G, Teige M, Metzlaff M, Hannah MA (2012) Chemical PARP inhibition enhances growth of Arabidopsis and reduces anthocyanin accumulation and the activation of stress protective mechanisms. PLoS ONE 7:e37284
Schulz P, Jansseune K, Degenkolbe T, Méret M, Claeys H, Skirycz A, Teige M, Willmitzer L, Hannah MA (2014) Poly(ADP-ribose)polymerase activity controls plant growth by promoting leaf cell number. PLoS ONE 9:e90322
Serrazina S, Machado H, Costa RL, Duque P, Malhó R (2021) Expression of castanea crenata allene oxide synthase in Arabidopsis improves the defense to Phytophthora cinnamomi. Front Plant Sci 12:149
Silva TF, Romanel EA, Andrade RR, Farinelli L, Østerås M, Deluen C, Corrêa RL, Schrago CE, Vaslin MF (2011) Profile of small interfering RNAs from cotton plants infected with the polerovirus Cotton leafroll dwarf virus. BMC Mol Biol 12(1):1–12
Stadler J, Richly H (2017) Regulation of DNA repair mechanisms: how the chromatin environment regulates the DNA damage response. Int J Mol Sci 18:1715
Tao Z, Gao P, Liu HW (2009) Studies of the expression of human poly(ADP-ribose) polymerase-1 in Saccharomyces cerevisiae and identification of PARP-1 substrates by yeast proteome microarray screening. Biochemistry 48:11745–11754
Tiwari R, Rajam MV (2022) RNA- and miRNA-interference to enhance abiotic stress tolerance in plants. J Plant Biochem Biotechnol. https://doi.org/10.1007/s13562-022-00770-9(0123456789
Tiwari R, Singh AK, Rajam MV (2022) Improved and reliable plant regeneration and Agrobacterium-mediated genetic transformation in soybean (Glycine max L.). J Crop Sci Biotechnol 2022:1–10
Travella S, Ross SM, Harden J, Everett C, Snape JW, Harwood WA (2005) A comparison of transgenic barley lines produced by particle bombardment and Agrobacterium-mediated techniques. Plant Cell Rep 23(12):780–789
Umesh DK, Pal M (2018) Differential role of jasmonic acid under drought and heat stress in rice (Oryza sativa). J Pharmacogn Phytochem 7:2626–2631
Vainonen JP, Shapiguzov A, Vaattovaara A, Kangasjärvi J (2016) Plant PARPs, PARGs and PARP-like proteins. Curr Protein Pept Sci 17(7):713–723
Vanderauwera S, De Block M, Van de Steene N, Van de Cotte B, Metzlaff M, Van Breusegem F (2007) Silencing of poly(ADP-ribose) polymerase in plants alters abiotic stress signal transduction. Proc Natl Acad Sci USA 104:15150–15155
Vargason JM, Szittya G, Burgyán J, Hall TMT (2003) Size selective recognition of siRNA by an RNA silencing suppressor. Cell 115(7):799–811
Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP (2007) Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3:12–23
Vaucheret H (2008) Plant argonautes. Trends Plant Sci 13(7):350–358
Wang YF, Dawson VL, Dawson TM (2009) Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp Neurol 218:93–202
Wang Y, Luo W, Wang Y (2019) PARP-1 and its associated nucleases in DNA damage response. DNA Repair 81:102651
Wasternack C, Feussner I (2018) The oxylipin pathways: biochemistry and function. Annu Rev Plant Biol 69:363–386
Willmitzer L (1979) Demonstration of in vitro covalent modification of chromosomal-proteins by poly(ADP) ribosylation in plant nuclei. FEBS Lett 108:13–16
Wilson C, Bellen HJ, Gehring WJ (1990) Position effects on eukaryotic gene expression. Annu Rev Cell Biol 6(1):679–714
Yao Q, Cong L, Chang JL, Li KX, Yang GX, He GY (2006) Low copy number gene transfer and stable expression in a commercial wheat cultivar via particle bombardment. J Exp Bot 57(14):3737–3746
Zhang D, Hu X, Li J, Liu J, Baks-te Bulte L, Wiersma M, Malik NUA, van Marion DM, Tolouee M, Hoogstra-Berends F, Lanters EA (2019) DNA damage-induced PARP1 activation confers cardiomyocyte dysfunction through NAD+ depletion in experimental atrial fibrillation. Nat Commun 10(1):1307
Zhou W, Li Y, Zhao BC, Ge RC, Shen YZ, Wang G, Huang ZJ (2009) Overexpression of TaSTRG gene improves salt and drought tolerance in rice. J Plant Physiol 166(15):1660–1671
Acknowledgements
We are grateful to the Indian Council of Agricultural Research, New Delhi (Grant F. No.: CS/18(14)/2015-O&P) for a research Grant on soybean. We also thank the Department of Biotechnology and the Department of Science and Technology, New Delhi, for their generous support for various research programmes in the lab. MVR is grateful to the University Grants Commission (UGC) for BSR Faculty Fellowship. RT is thankful to the UGC for Senior Research Fellowship. We also thank the UGC for SAP (DRS-III) programme, DST for FIST (Level 2) programme, and DU-DST PURSE (Phase II) Grant.
Funding
This work was generously supported by the Indian Council of Agricultural Research, New Delhi (Grant F. No.: CS/18(14)/2015-O&P).
Author information
Authors and Affiliations
Contributions
MVR and AKS has conceived the concept. MVR, AKS and RT designed the experiments, and RT performed the experiments, generated and analysed the data, and wrote the manuscript. MVR and RT revised the manuscript and interpreted the data. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Ravinder Kumar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10725_2023_1047_MOESM2_ESM.jpg
Supplementary file2 (JPG 717 kb). Morphology of putative soybean T0 transformants developed using GmPARP1- and GmPARP2-RNAi constructs
10725_2023_1047_MOESM3_ESM.jpg
Supplementary file3 (JPG 750 kb). Fig. S3 PCR analysis of putative soybean GmPARP1-RNAi T0 transformants. (A) PCR analysis of marker gene using bar primers (~407 bp). Lane M. 1 kb/100 bp ladder, Lane N. Negative control, Lane UT. Untransformed control, Lane 1-25. Putative soybean PARP1-RNAi T0 transformants; (B) PCR analysis of gene of interest using CaMV 35SP forward primer and PARP1 sense as a reverse primer (~392 bp). Lane M. 100 bp ladder, Lane N. Negative control, Lane UT. Untransformed control, Lane 1-25. Putative soybean PARP1-RNAi T0 transformants
10725_2023_1047_MOESM4_ESM.jpg
Supplementary file4 (JPG 373 kb). Fig. S4 PCR analysis of putative soybean GmPARP2-RNAi T0 transformants. (A) PCR analysis of marker gene using bar primers (~407 bp). Lane M. 100 bp ladder, Lane N. Negative control, Lane UT. Untransformed control, Lane 2-23. Putative soybean PARP2-RNAi T0 transformants; (B) PCR analysis of gene of interest using CaMV 35SP forward primer and PARP2 sense as a reverse primer (~393bp). Lane M. 100 bp ladder, Lane N. Negative control, Lane UT. Untransformed control, Lane 2-23. Putative soybean PARP2-RNAi T0 transformants
10725_2023_1047_MOESM5_ESM.jpg
Supplementary file5 (JPG 426 kb). Fig. S5 Southern analysis using bar gene-specific probe. (A and B) corresponds to Southern hybridization using GmPARP1-RNAi T0 transformants and 9, 10, 14, 15, 16, 17, 18, 19, 20 represent independent T0 transformants; (C) corresponds to Southern hybridization using GmPARP2-RNAi T0 transformants and 2, 3, 4, 6, 8, 11, 20, 21, 23 represent independent T0 transformants. UT corresponds to untransformed control, P corresponds to pFGC5941 vector as a positive control
10725_2023_1047_MOESM6_ESM.jpg
Supplementary file6 (JPG 407 kb). Fig. S6 Molecular analysis of soybean GmPARP1-RNAi T1 transgenics. (A) PCR analysis of marker gene using bar primers (~407 bp). Lane M. 100 bp ladder, Lane N. Negative control, Lane UT. Untransformed control, Lane 9.1-19.3. Putative soybean GmPARP1-RNAi T1 transgenic lines; (B) PCR analysis of gene of interest using CaMV 35SP forward primer and PARP1 sense as a reverse primer (~392 bp). Lane M. 100 bp ladder, Lane 1. Negative control, Lane 2. Untransformed control, Lane 9.1-19.3. Putative soybean GmPARP1-RNAi T1 transgenic lines
10725_2023_1047_MOESM7_ESM.jpg
Supplementary file7 (JPG 451 kb). Fig. S7 Molecular analysis of soybean GmPARP2-RNAi T1 transgenics. (A) PCR analysis of marker gene using bar primers (~407 bp). Lane M. 100 bp ladder, Lane N. Negative control, Lane UT. Untransformed control, Lane 6.1-21.35. Putative soybean GmPARP2-RNAi T1 transgenic lines; (B) PCR analysis using CaMV 35SP forward primer and PARP2 sense as a reverse primer (~393bp). Lane M. 100 bp ladder, Lane N. Negative control, Lane UT. Untransformed control, Lane 6.1-21.35. Putative soybean GmPARP2-RNAi T1 transgenic lines
10725_2023_1047_MOESM8_ESM.jpg
Supplementary file8 (JPG 194 kb). Fig. S8 Standard curve of proline prepared from a serial dilution of 100 ppm stock solution of L-Proline
10725_2023_1047_MOESM9_ESM.docx
Supplementary file9 (DOCX 14 kb). Table S1 Details of the primers used in gene amplification, transgene expression analysis and siRNA detection
10725_2023_1047_MOESM10_ESM.docx
Supplementary file10 (DOCX 13 kb). Table S2 List of primers used in RT-qPCR expression analysis and stress-associated gene expression
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tiwari, R., Singh, A.K. & Rajam, M.V. GmPARPs differentially regulate the drought and heat stress tolerance in soybean. Plant Growth Regul 101, 643–661 (2023). https://doi.org/10.1007/s10725-023-01047-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-01047-4