Abstract
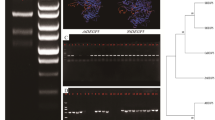
Antimicrobial peptides (AMPs) play a pivotal role in plant immune responses to diverse stresses, and hence, have become the novel molecules for studying plant responses to environmental harsh conditions. For the first time, the effect of the antimicrobial peptide defensin NaD1 on drought stress was investigated in tobacco by generating the NaD1 transgenic lines overexpressing the coding sequence of NaD1. The NaD1 expression was confirmed by RT-PCR, and the NaD1 peptide presence was verified using ELISA and western blot analysis in the tobacco transgenic lines. In silico bioinformatic analysis revealed that the most abundant components in Cis-regulatory elements in NaD1 homologs in Nicotiana attenuate (NaDEF genes) are MYB, MYC and ABRE elements suggesting that the NaD1 promoter is involved in the abiotic stress regulation. The chlorophyll a, b, and total chlorophyll contents, and correspondingly, the index of chlorophyll stability (ICS) significantly (P ≤ 0.01) increased in all 3 NaD1 transgenic lines under drought stress. Moreover, activities of Catalase (CAT), Peroxidase (POD), Ascorbate peroxidase (APX) and Superoxide dismutase (SOD) were significantly enhanced in response to drought stress in the transgenic lines. Among the three transgenic lines, line 1 showed more tolerance to the drought stress. The data together suggest that the expression of NaD1 increases the antioxidant activity of the enzymes presumably leading to elimination of ROS levels and maintenance of the chlorophyll content and stability, resulting in enhanced drought tolerance in the transgenic tobacco lines. Therefore, the defensin NaD1 is an essential factor in regulation of plant responses to drought stress.








Similar content being viewed by others

Change history
27 July 2023
The title of the article has been updated.
References
Ajithkumar IP, Panneerselvam R (2014) ROS scavenging system, osmotic maintenance, pigment and growth status of Panicum sumatrense roth. Under drought stress. Cell Biochem Biophys 68(3):587–595
Allen A, Snyder AK, Preuss M, Nielsen EE, Shah DM, Smith TJ (2008) Plant defensins and virally encoded fungal toxin KP4 inhibit plant root growth. Planta 227(2):331–339. https://doi.org/10.1007/s00425-007-0620-1
Arazmjo A, Heidari M, Ghanbari A (2010) The effect of water stress and three sources of fertilizers on flower yield, physiological parameters and nutrient uptake in chamomile (Matricaria chamomilla L). Iran J Med Aromatic Plants 25(4):482–494
Badrhadad A, Nazarian-Firouzabadi F, Ismaili A (2018) Fusion of a chitin-binding domain to an antibacterial peptide to enhance resistance to Fusarium solani in tobacco (Nicotiana tabacum). 3 Biotech 8(9):391
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Bleackley MR, Payne JA, Hayes BM, Durek T, Craik DJ, Shafee TM, Poon IK, Hulett MD, van der Weerden NL, Anderson MA (2016) Nicotiana alata defensin chimeras reveal differences in the mechanism of fungal and Tumor Cell Killing and an enhanced antifungal variant. Antimicrob Agents Chemother 60(10):6302–6312. https://doi.org/10.1128/AAC.01479-16
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Campo S, Manrique S, Garcia-Martinez J, San Segundo B (2008) Production of cecropin A in transgenic rice plants has an impact on host gene expression. Plant Biotechnol J 6(6):585–608. https://doi.org/10.1111/j.1467-7652.2008.00339.x
Chance B, Maehly A (1955) [136] Assay of catalases and peroxidases
Chen S-C, Liu A-R, Zou Z-R (2006) Overexpression of glucanase gene and defensin gene in transgenic tomato enhances resistance to Ralstonia solanacearum. Russ J Plant Physiol 53(5):671–677
Chen S, Zhao H, Luo T, Liu Y, Nie X, Li H (2019) Characteristics and expression pattern of MYC genes in Triticum aestivum, Oryza sativa, and Brachypodium distachyon. Plants 8(8):274
De Vos M, Van Oosten VR, Van Poecke RM, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux J-P, Van Loon L, Dicke M (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18(9):923–937
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot 32(1):79–91
Do HM, Lee SC, Jung HW, Sohn KH, Hwang BK (2004) Differential expression and in situ localization of a pepper defensin (CADEF1) gene in response to pathogen infection, abiotic elicitors and environmental stresses in Capsicum annuum. Plant Sci 166(5):1297–1305
Esfandiari E, Shekari F, Shekari F, Esfandiari M (2007) The effect of salt stress on antioxidant enzymes’activity and lipid peroxidation on the wheat seedling. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 35(1):48
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S (2009) Plant drought stress: effects, mechanisms and management. Sustainable agriculture. Springer, pp 153–188
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Holaskova E, Galuszka P, Frebort I, Oz MT (2015) Antimicrobial peptide production and plant-based expression systems for medical and agricultural biotechnology. Biotechnol Adv 33(6 Pt 2):1005–1023. https://doi.org/10.1016/j.biotechadv.2015.03.007
Isah T (2019) Stress and defense responses in plant secondary metabolites production. Biol Res 52
Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Do Choi Y, Cheong J-J (2008) Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol 146(2):623
Karpun N, Yanushevskaya E, Mikhailova YV (2015) Formation of plants nonspecific induced immunity at the biogenous stress. СельскоÑ озяйственная биология 5(eng):540–549
Khademi M, Varasteh-Shams M, Nazarian-Firouzabadi F, Ismaili A (2020) New recombinant antimicrobial peptides confer resistance to fungal pathogens in tobacco plants. Front Plant Sci 11:1236
Kiani SP, Maury P, Sarrafi A, Grieu P (2008) QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well-watered and water-stressed conditions. Plant Sci 175(4):565–573
Koike M, Okamoto T, Tsuda S, Imai R (2002) A novel plant defensin-like gene of winter wheat is specifically induced during cold acclimation. Biochem Biophys Res Commun 298(1):46–53
Koul A, Sharma D, Kaul S, Dhar MK (2019) Identification and in silico characterization of cis-acting elements of genes involved in carotenoid biosynthesis in tomato. 3 Biotech 9:1–11
Kumar M, Yusuf MA, Yadav P, Narayan S, Kumar M (2019) Overexpression of chickpea defensin gene confers tolerance to water-deficit stress in Arabidopsis thaliana. Front Plant Sci 10:290
Lacerda AF, Vasconcelos EA, Pelegrini PB, Grossi de Sa MF (2014) Antifungal defensins and their role in plant defense. Front Microbiol 5:116. https://doi.org/10.3389/fmicb.2014.00116
Lay F, Anderson M (2005) Defensins-components of the innate immune system in plants. Curr Protein Pept Sci 6(1):85–101
Lay FT, Brugliera F, Anderson MA (2003) Isolation and properties of floral defensins from ornamental tobacco and petunia. Plant Physiol 131(3):1283–1293. https://doi.org/10.1104/pp.102.016626
Lee IH, Jung YJ, Cho YG, Nou IS, Huq MA, Nogoy FM, Kang KK (2017) SP-LL-37, human antimicrobial peptide, enhances disease resistance in transgenic rice. PLoS ONE 12(3):e0172936. https://doi.org/10.1371/journal.pone.0172936
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30(1):325–327. https://doi.org/10.1093/nar/30.1.325
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Mohan M, Narayanan SL, Ibrahim S (2000) Chlorophyll stability index (CSI): its impact on salt tolerance in rice. Int Rice Res Notes 25(2):38–39
Nakano Y, Asada K (1987) Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol 28(1):131–140
Nongpiur R, Soni P, Karan R, Singla-Pareek SL, Pareek A (2012) Histidine kinases in plants: cross talk between hormone and stress responses. Plant Signal Behav 7(10):1230–1237
Olga K, Marina K, Alexey A, Anton S, Vladimir Z, Igor T (2020) The role of plant antimicrobial peptides (AMPs) in response to biotic and abiotic environmental factors. Biol Commun 65(2):187–199
Pang S-Z, Rasmussen J, Ye G-N, Sanford JC (1992) Use of the signal peptide of Pisum vicilin to translocate β-glucuronidase in Nicotiana tabacum. Gene 112(2):229–234
Poon I, Baxter AA, Lay FT, Mills GD, Adda CG, Payne JA, Phan TK, Ryan GF, White JA, Veneer PK, van der Weerden NL, Anderson MA, Kvansakul M, Hulett MD (2014) Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. Elife 3:e01808. https://doi.org/10.7554/eLife.01808
Qiu Z, Guo J, Zhu A, Zhang L, Zhang M (2014) Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol Environ Saf 104:202–208. https://doi.org/10.1016/j.ecoenv.2014.03.014
Richards E, Reichardt M, Rogers S (1994) Preparation of genomic DNA from plant tissue. Curr Protoc Mol Biol 27(1):2 1-2.3. 7
Russell DW, Sambrook J (2001) Molecular cloning: a laboratory manual, vol 1. Cold Spring Harbor Laboratory Cold Spring Harbor, NY
Sabokkhiz MA, Tanhaeian A, Mamarabadi M (2019) Study on antiviral activity of two recombinant antimicrobial peptides against Tobacco Mosaic Virus. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-019-09539-4
Sairam R, Deshmukh P, Saxena D (1998) Role of antioxidant systems in wheat genotypes tolerance to water stress. Biol Plant 41(3):387–394
Sharma S, Sanyal SK, Sushmita K, Chauhan M, Sharma A, Anirudhan G, Veetil SK, Kateriya S (2021) Modulation of phototropin signalosome with artificial illumination holds great potential in the development of climate-smart crops. Curr Genom 22(3):181
Sher Khan R, Iqbal A, Malak R, Shehryar K, Attia S, Ahmed T, Ali Khan M, Arif M, Mii M (2019) Plant defensins: types, mechanism of action and prospects of genetic engineering for enhanced disease resistance in plants. 3 Biotech 9(5):192. https://doi.org/10.1007/s13205-019-1725-5
Stolf-Moreira R, Medri M, Neumaier N, Lemos N, Pimenta J, Tobita S, Brogin R, Marcelino-Guimarães F, Oliveira M, Farias J (2010) Soybean physiology and gene expression during drought. Genet Mol Res 9(4):1946–1956
Sui J, Jiang D, Zhang D, Song X, Wang J, Zhao M, Qiao L (2016) The salinity responsive mechanism of a hydroxyproline-tolerant mutant of peanut based on digital gene expression profiling analysis. PLoS ONE 11(9):e0162556
Tahkokorpi M, Taulavuori K, Laine K, Taulavuori E (2007) After-effects of drought-related winter stress in previous and current year stems of Vaccinium myrtillus L. Environ Exp Bot 61(1):85–93
Tam JP, Wang S, Wong KH, Tan WL (2015) Antimicrobial peptides from plants. Pharmaceuticals 8(4):711–757
Wang X, Niu Y, Zheng Y (2021) Multiple functions of MYB transcription factors in abiotic stress responses. Int J Mol Sci 22(11):6125
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144(3):307–313
Ye Y, Ding Y, Jiang Q, Wang F, Sun J, Zhu C (2017) The role of receptor-like protein kinases (RLKs) in abiotic stress response in plants. Plant Cell Rep 36(2):235–242
Zhang H, Sonnewald U (2017) Differences and commonalities of plant responses to single and combined stresses. Plant J 90(5):839–855
Acknowledgements
This research was carried out with partially financial assistance of Iran National Science Foundation (INSF) under number of 98022316. This study was financially supported by grant NO: biodc-13085-24100.1 of the Biotechnology Development Council of the Islamic Republic of Iran.
Funding
This research was partly funded by University of Guilan, this research was carried out with partially financial assistance of Iran National Science Foundation (INSF) under number of 98022316. This study was financially supported by grant NO: biodc-13085-24100.1 of the Biotechnology Development Council of the Islamic Republic of Iran.
Author information
Authors and Affiliations
Contributions
SR did the research experiments, data analysis and wrote the first draft of manuscript. RSH conducted and supervised the whole research project and revised the manuscript. AZ, MBN and FNF cooperate as advisors in different parts of the work, respectively. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Neelam sangwan.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“The original online version of this article was revised”: The title of the article has been revised.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Royan, S., Shirzadian-Khorramabad, R., Zibaee, A. et al. Tobacco plants expressing the defensin NaD1 enhance drought tolerance characteristics in transgenic lines. Plant Growth Regul 101, 503–518 (2023). https://doi.org/10.1007/s10725-023-01037-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-01037-6