Abstract
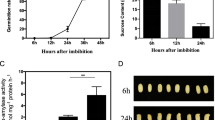
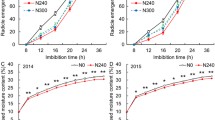
Seeds can activate a series of genes to avoid imbibition-associated stress during seed germination. However, the precise gene responses at the initial imbibition stage (Phase I) of seed germination are not yet fully understood in rice. In this study, a total of 1544 differentially expressed genes (DEGs) with at least 2-fold change were identified in 8 h imbibed seeds (Phase I) compared to dry seeds of rice using RNA-Seq approach. MapMan analysis revealed that the mainly signalling-, cell wall-, abiotic stress-, and antioxidant-related DEGs were associated with stress responses pathway involving in the initial imbibition of rice seed germination. Among them, the signalling-related DEGs were mainly receptor kinases, and the largest number of cell wall-related DEGs were expansins followed by pectinesterases and polygalacturonases. The abiotic stress-related DEGs were mainly cupin domain protein, methyltransferases and SPX domain protein, and the majority of antioxidant-related DEGs were glutathione S-transferases (GSTs) and peroxidases. Further qRT-PCR analysis revealed that the highest expressions of the majority of GST genes occurred at 8 h imbibition stage in rice, which caused the corresponding highest GST activity at that stage. GSTs might prevent the burst of H2O2 accumulation at the initial imbibition stage that contributes to the following successful seed germination. Our results provide further understanding of gene responses at the initial imbibition stage of seed germination in rice. The identified genes provide a foundation for future studies of seed germination in rice.









Similar content being viewed by others
Data availability
The RNA sequencing data have been submitted to the Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra/) under accession number PRJNA544406.
References
An YQ, Lin L (2011) Transcriptional regulatory programs underlying barley germination and regulatory functions of gibberellin and abscisic acid. BMC Plant Biol 11:105
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331:806–814
Basbouss-Serhal I, Leymarie J, Bailly C (2016) Fluctuation of Arabidopsis seed dormancy with relative humidity and temperature during dry storage. J Exp Bot 67:119–130
Bewley JD, Bradford KJ, Hilhorst HW, Nonogaki H (2013) Seeds: physiology of development, germination and dormancy, 3rd edn. Springer, Heidelberg, pp 145–147
Chen JH, Jiang HW, Hsieh EJ, Chen HY, Chien CT, Hsieh HL, Lin TP (2012) Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol 158:340–351
Cheng X, Wu Y, Guo J, Du B, Chen R, Zhu L, He G (2013) A rice lectin receptor-like kinase that is involved in innate immune responses also contributes to seed germination. Plant J 76:687–698
Cheng J, Wang L, Zeng P, He Y, Zhou R, Zhang H, Wang Z (2017) Identification of genes involved in rice seed priming in the early imbibition stage. Plant Biol 19:61–69
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Dametto A, Sperotto RA, Adamski JM, Blasi ÉA, Cargnelutti D, de Oliveira LF, Ricachenevsky FK, Fregonezi JN, Mariath JE, da Cruz RP, Margis R, Fett JP (2015) Cold tolerance in rice germinating seeds revealed by deep RNAseq analysis of contrasting indica genotypes. Plant Sci 238:1–12
Delteil A, Gobbato E, Cayrol B, Estevan J, Michel-Romiti C, Dievart A, Kroj T, Morel JB (2016) Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol 16:17
Deng K, Wang Q, Zeng J, Guo X, Zhao X, Tang D, Liu X (2009) A lectin receptor kinase positively regulates ABA response during seed germination and is involved in salt and osmotic stress response. J Plant Biol 52:493–500
Davidson RM, Manosalva PM, Snelling J, Bruce M, Leung H, Leach JE (2010) Rice germin-like proteins: allelic diversity and relationships to early stress responses. Rice 3:43–55
Dixon DP, Lapthorn A, Edwards R (2002) Plant glutathione transferases. Genome Biol 3:REVIEWS3004
Endo A, Tatematsu K, Hanada K, Duermeyer L, Okamoto M, Yonekura-Sakakibara K, Saito K, Toyoda T, Kawakami N, Kamiya Y, Seki M, Nambara E (2012) Tissue-specific transcriptome analysis reveals cell wall metabolism, flavonol biosynthesis and defense responses are activated in the endosperm of germinating Arabidopsis thaliana seeds. Plant Cell Physiol 53:16–27
Gao LL, Xue HW (2012) Global analysis of expression profiles of rice receptor-like kinase genes. Mol Plant 5:143–153
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine. J Free Radic Biol Med 1:331–332
Han C, Yang P (2015) Studies on the molecular mechanisms of seed germination. Proteomics 15:1671–1679
He D, Yang P (2013) Proteomics of rice seed germination. Front Plant Sci 4:246
He D, Han C, Yao J, Shen S, Yang P (2011) Constructing the metabolic and regulatory pathways in germinating rice seeds through proteomic approach. Proteomics 11:2693–2713
He Y, Cheng J, He Y, Yang B, Cheng Y, Yang C, Zhang H, Wang Z (2019) Influence of isopropylmalate synthase OsIPMS1 on seed vigour associated with amino acid and energy metabolism in rice. Plant Biotechnol J 17:322–337
Holdsworth MJ, Bentsink L, Soppe WJ (2008) Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. N Phytol 179:33–54
Houston K, Tucker MR, Chowdhury J, Shirley N, Little A (2016) The plant cell wall: a complex and dynamic structure as revealed by the responses of genes under stress conditions. Front Plant Sci 7:984
Jimenez A, Hernandez JA, Del Rio LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114:275–284
Karmous I, Trevisan R, El Ferjani E, Chaoui A, Sheehan D (2017) Redox biology response in germinating Phaseolus vulgaris seeds exposed to copper: evidence for differential redox buffering in seedlings and cotyledon. PLoS ONE 12:e0184396
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14:R36
Kumar JSP, Prasad RS, Banerjee R, Thammineni C (2015) Seed birth to death: dual actions of reactive oxygen species in seed physiology. Ann Bot 116:663–668
Le Gall H, Philippe F, Domon JM, Gillet F, Pelloux J, Rayon C (2015) Cell wall metabolism in response to abiotic stress. Plants 4:112–166
Leucci MR, Lenucci MS, Piro G, Dalessandro G (2008) Water stress and cell wall polysaccharides in the apical root zone of wheat cultivars varying in drought tolerance. J Plant Physiol 165:1168–1180
Li WY, Chen BX, Chen ZJ, Gao YT, Chen Z, Liu J (2017) Reactive oxygen species generated by NADPH oxidases promote radicle protrusion and root elongation during rice seed germination. Int J Mol Sci 18:110
Liu H, Ma Y, Chen N, Guo S, Liu H, Guo X, Chong K, Xu Y (2014) Overexpression of stress-inducible OsBURP16, the β subunit of polygalacturonase 1, decreases pectin content and cell adhesion and increases abiotic stress sensitivity in rice. Plant Cell Environ 37:1144–1158
Liu SJ, Xu HH, Wang WQ, Li N, Wang WP, Møller IM, Song SQ (2015) A proteomic analysis of rice seed germination as affected by high temperature and ABA treatment. Physiol Plant 154:142–161
Liu SJ, Xu HH, Wang WQ, Li N, Wang WP, Lu Z, Møller IM, Song SQ (2016) Identification of embryo proteins associated with seed germination and seedling establishment in germinating rice seeds. J Plant Physiol 196–197:79–92
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2– DDC(T) method. Methods 25:402–408
Lou Q, Chen L, Sun Z, Xing Y, Li J, Xu X, Mei H, Luo L (2007) A major QTL associated with cold tolerance at seedling stage in rice (Oryza sativa L.). Euphytica 158:87–94
Macovei A, Pagano A, Leonetti P, Carbonera D, Balestrazzi A, Araujo SS (2017) Systems biology and genome-wide approaches to unveil the molecular players involved in the pre-germinative metabolism: implications on seed technology traits. Plant Cell Rep 36:669–688
Mccormac AC, Keefe PD (1990) Cauliflower (Brassica oleracea L.) seed vigour: imbibition effects. J Exp Bot 41:893–899
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Morillo SA, Tax FE (2006) Functional analysis of receptor-like kinases in monocots and dicots. Curr Opin Plant Biol 9:460–469
Morris ER, Walker JC (2003) Receptor-like protein kinases: the keys to response. Curr Opin Plant Biol 6:339–342
Müller K, Levesque-Tremblay G, Bartels S, Weitbrecht K, Wormit A, Usadel B, Haughn G, Kermode AR (2013) Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol 161:305–316
Novaković L, Guo T, Bacic A, Sampathkumar A, Johnson KL (2018) Hitting the wall-sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants (Basel) 7:E89
Oracz K, Karpiński S (2016) Phytohormones signaling pathways and ROS involvement in seed germination. Front Plant Sci 7:864
Osakabe Y, Maruyama K, Seki M, Satou M, Shinozaki K, Yamaguchi-Shinozaki K (2005) Leucine-rich repeat receptor-like kinase 1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis Plant Cell 17:1105–1119
Osakabe Y, Mizuno S, Tanaka H, Maruyama K, Osakabe K, Todaka D, Fujita Y, Kobayashi M, Shinozaki K, Yamaguchi-Shinozaki K (2010) Overproduction of the membrane-bound receptor-like protein kinase 1, RPK1, enhances abiotic stress tolerance in Arabidopsis J Biol Chem 285:9190–9201
Sreenivasulu N, Usadel B, Winter A, Radchuk V, Scholz U, Stein N, Weschke W, Strickert M, Close TJ, Stitt M, Graner A, Wobus U (2008) Barley grain maturation and germination: metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol 146:1738–1758
Srivastava D, Verma G, Chauhan AS, Pande V, Chakrabarty D (2019) Rice (Oryza sativa L.) tau class glutathione S-transferase (OsGSTU30) overexpression in Arabidopsis thaliana modulates a regulatory network leading to heavy metal and drought stress tolerance. Metallomics 11:375–389
Steinwand BJ, Kieber JJ (2010) The role of receptor-like kinases in regulating cell wall function. Plant Physiol 153:479–484
Su L, Lan Q, Pritchard HW, Hua X, Wang X (2016) Reactive oxygen species induced by cold stratification promote germination of Hedysarum scoparium seeds. Plant Physiol Biochem 109:406–415
Tenhaken R (2015) Cell wall remodeling under abiotic stress. Front Plant Sci 5:771
Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37:914–939
Usadel B, Poree F, Nagel A, Lohse M, Czedik-Eysenberg A, Stitt M (2009) A guide to using MapMan to visualize and compare Omics data in plants: a case study in the crop species, Maize. Plant Cell Environ 32:1211–1229
Vaid N, Macovei A, Tuteja N (2013) Knights in action: lectin receptor-like kinases in plant development and stress responses. Mol Plant 6:1405–1418
Ventura L, Donà M, Macovei A, Carbonera D, Buttafava A, Mondoni A, Rossi G, Balestrazzi A (2012) Understanding the molecular pathways associated with seed vigor. Plant Physiol Biochem 60:196–206
Wang C, Wei Q, Zhang K, Wang L, Liu F, Zhao L, Tan Y, Di C, Yan H, Yu J, Sun C, Chen WJ, Xu W, Su Z (2013) Down-regulation of OsSPX1 causes high sensitivity to cold and oxidative stresses in rice seedlings. PLoS ONE 8:e81849
Wang WQ, Liu SJ, Song SQ, Moller IM (2015) Proteomics of seed development, desiccation tolerance, germination and vigor. Plant Physiol Biochem 86:1–15
Wang Z, Ruan W, Shi J, Zhang L, Xiang D, Yang C, Li C, Wu Z, Liu Y, Yu Y, Shou H, Mo X, Mao C, Wu P (2014) Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc Natl Acad Sci USA 111:14953–14958
Wang Z, Wang J, Bao Y, Wu Y, Zhang H (2011) Quantitative trait loci controlling rice seed germination under salt stress. Euphytica 178:297–307
Wei T, He Z, Tan X, Liu X, Yuan X, Luo Y, Hu S (2015) An integrated RNA-Seq and network study reveals a complex regulation process of rice embryo during seed germination. Biochem Biophys Res Commun 464:176–181
Xu J, Zheng AQ, Xing XJ, Chen L, Fu XY, Peng RH, Tian YS, Yao QH (2018) Transgenic Arabidopsis plants expressing grape glutathione S-transferase gene (VvGSTF13) show enhanced tolerance to abiotic stress. Biochemistry 83:755–765
Xu E, Chen M, He H, Zhan C, Cheng Y, Zhang H, Wang Z (2017) Proteomic analysis reveals proteins involved in seed imbibition under salt stress in rice. Front Plant Sci 7:2006
Yang G, Xu Z, Peng S, Sun Y, Jia C, Zhai M (2016) In planta characterization of a tau class glutathione S-transferase gene from Juglans regia (JrGSTTau1) involved in chilling tolerance. Plant Cell Rep 35:681–692
Yang P, Li X, Wang X, Chen H, Chen F, Shen S (2007) Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics 7:3358–3368
Zhang XY, Nie ZH, Wang WJ, Leung DW, Xu DG, Chen BL, Chen Z, Zeng LX, Liu EE (2013) Relationship between disease resistance and rice oxalate oxidases in transgenic rice. PLoS ONE 8:e78348
Acknowledgements
This work was supported by the National Key Research and Development Plan (Grant No. 2018YFD0100901), the Guangdong Province Key Research and Development Program (Grant No. 2018B020202012), the National Natural Science Foundation of China (Grant No. 31771889), the Guangdong Province Key Laboratory of Plant Molecular Breeding (Grant No. GPKLPMB201903), and the Major Scientific Research Projects of General Colleges and Universities of Guangdong Province (Grant No. 2017KTSCX024).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, J., He, Y., Li, X. et al. An integrated RNA-Seq and physiological study reveals gene responses involving in the initial imbibition of seed germination in rice. Plant Growth Regul 90, 249–263 (2020). https://doi.org/10.1007/s10725-019-00567-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-019-00567-2