Abstract
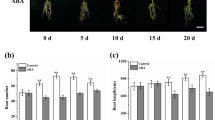
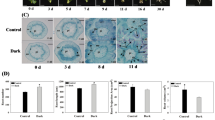
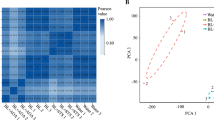
Malus hupehensis seedlings were treated with exogenous applications of 0.5 mg L−1 brassinolide (BR) in order to investigate the mechanism by which BR promotes and effects lateral root development. Root morphology, plant height, plant crown diameter, and endogenous levels of Brassinolide (BR), Auxin (IAA), Abscisic acid (ABA), and Gibberellin (GA3) in lateral roots were evaluated. Additionally, differential gene expression of genes related to root development were examined in treated and untreated roots by RT-qPCR during lateral root development. Results indicated that BR treatment promoted both root and shoot growth and auxin levels increased, while ABA and GA3 declined. Additionally, there was also a significant upregulation in the expression of MdBAK1, MdBRI1, and MdBZR1 in response to the BR treatment. Transcript levels of key auxin synthesis and transport genes, such as MdYUCCA6, MdYUCCA10, MdPIN1, MdPIN2, MdPIN3, which corresponded with higher auxin levels in the BR-treated samples. MdIAA23 were induced, but the expression of MdIAA5, a negative regulator of MdARF7 and MdARF19, was downregulated in BR-treated apple seedlings, leading to an elevated expression of MdARRO1; which in turn increased lateral root development. On the other hand, MdBRI1 induced MdWOX5 expression, resulting in enhanced expression of the cell cycle related genes: MdCYCD1;1, MdCYCD3;1, and MdCYCD3;2; leading to upregulated expression of MdLBD29. Collectively, the changes in gene expression and hormone levels resulted in an increased number of lateral roots, and other growth characteristics in BR-treated plants.





Similar content being viewed by others
References
Artlip TS (2016) An apple rootstock overexpressing a peach CBF gene alters growth and flowering in the scion but does not impact cold hardiness or dormancy. Hortic Res 3:16006
Bao F, Shen J, Muday GK et al (2004) Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol 134(4):1624–1631
Bergonci T, Ribeiro B, Ceciliato PH, Guerreroabad JC, Silvafilho, M C, Moura DS (2014) Arabidopsis thaliana ralf1 opposes brassinosteroid effects on root cell elongation and lateral root formation. J Exp Bot 65(8):2219–2230
Brady SM, Sarkar SF, Bonetta D, Mc Court P (2003) The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J 34(1):67–75
Chaiwanon J, and Wang Z Y (2015) Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in Arabidopsis roots. Curr Biol Cb 25(8):1031–1042
Chen CW, Yang YW, Lur HS, Tsai YG, Chang MC (2006) A novel function of abscisic acid in the regulation of rice (Oryza sativa L.) root growth and development. Plant Cell Physiol 47(1):1–13
Cheng Y, Dai X, Zhao Y (2006) Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20(13):1790–1799
Clouse SD, Langford M, McMorris TC (1996) A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol 111(3):671–678
Cui H, Hao Y, Kovtun M et al (2011) Genome-wide direct target analysis reveals a role for SHORT-ROOT in root vascular patterning through cytokinin homeostasis. Plant Physiol 157(3):1221–1231
Depuydt S, Hardtke CS (2011) Hormone signalling crosstalk in plant growth regulation. Curr Biol 21(9):R365–R373
Ding J, Mao LJ, Wang ST et al (2013) Determination of endogenous brassinosteroids in plant tissues using solid-phase extraction with double layered cartridge followed by High-performance liquid chromatography–tandem mass spectrometry. Phytochem Anal 24(4):386–394
Dobrev PI, Kamínek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr A 950(1–2):21–29
Dockter C, Gruszka D, Braumann I et al (2014) Induced variations in brassinosteroid genes define barley height and sturdiness, and expand the green revolution genetic toolkit. Plant Physiol 166(4):1912–1927
Feng Z, Sun X, Wang G et al (2012) LBD29 regulates the cell cycle progression in response to auxin during lateral root formation in Arabidopsis thaliana. Ann Bot (Lond) 110(1):1–10
Ferreira P C G, Hemerly AS, de Almeida Engler L, Van Montagu M, Engler G, InzéD (1994) Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell 6(12):1763–1774
Forzani C, Aichinger E, Sornay E, Willemsen V, Laux T, Dewitte W, Murray JA (2014) WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche. Curr Biol 24(16):1939–1944
Gambino G, Perrone I, Gribaudo I (2008) A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal 19(6):520–525
Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD (2008) Cell-specific nitrogen responses mediate developmental plasticity. Proc Natl Acad Sci USA 105(2):803–808
González-García M P, Vilarrasablasi J, Zhiponova M et al (2011) Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 138(5):849–859
Gou J, Strauss SH, Tsai CJ, Fang K, Chen Y, Jiang X, Busov VB (2010) Gibberellin regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell 22(3):623–639
Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCF TIR1-dependent degradation of AUX/IAA proteins. Nature 414(6861):271–276
Grieneisen VA, Xu J, Marée A F M, Hogeweg P, Scheres B (2007) Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449(7165):1008–1013
Guo H, Li L, Aluru M et al (2013) Mechanisms and networks for brassinosteroid regulated gene expression. Curr Opin Plant Biol 16(5):545–553
Gutierrez L, Bussell JD, Păcurar DI et al (2009) Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 21(10):3119–3132
He K, Gou X, Yuan T et al (2007) BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17(13):1109–1115
Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN (2000) The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101(5):555–567
Kauschmann A, Jessop A, Koncz C et al (1996) Genetic evidence for an essential role of brassinosteroids in plant development. Plant J 9(5):701–713
Kinoshita N, Wang H, Kasahara H et al (2012) IAA-Ala Resistant3, an evolutionarily conserved target of miR167, mediates Arabidopsis root architecture changes during high osmotic stress. Plant Cell 24(9):3590–3602
Kong DY, Yue I, Hong C (2016) The WUSCHEL related homeobox protein WOX7 regulates the sugar response of lateral root development in Arabidopsis thaliana. Mol Plant 9(2):261–270
Kuppusamy KT, Chen AY, Nemhauser JL (2009) Steroids are required for epidermal cell fate establishment in Arabidopsis roots. Proc Natl Acad Sci USA 106(19):8073–8076
Latha P, Vardhini B (2016) Effect of brassinolide on the growth of mustard crops grown in semi-arid tropics of Nizamabad. Int J Plant Soil Sci 9(1):1–5
Lee SH, Kim Y, Pham G et al (2015) Brassinazole resistant 1 (BZR1)-dependent brassinosteroid signalling pathway leads to ectopic activation of quiescent cell division and suppresses columella stem cell differentiation. J Exp Bot 66(15):4835–4849
Li J, Wen J, Lease KA et al (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signalling. Cell 110(2):213–222
Li L, Xu J, Xu ZH et al (2005) Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in Brassica and Arabidopsis. Plant Cell 17(10):2738–2753
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT Method. Methods 25(4):402–408
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124(1):33
McKay MJ, Ross JJ, Lawrence NL et a1 (1994) Control ofinternode length in pisumsativum further evidence for the involvement of indole-3.acetic acid. Plant Physiol 106(4):1521–1526
Mimida N, Kidou S, Iwanami H et al (2011) Apple FLOWERING LOCUS T proteins interact with transcription factors implicated in cell growth and organ development. Tree Physiol 31(31):555–566
Mouchel CF, Osmont KS, Hardtke CS (2006) BRX mediates feedback between brassinosteroid levels and auxin signalling in root growth. Nature 443(7110):458–461
Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17(23):6903–6911
Müssig C, Shin GH, Altmann T (2003) Brassinosteroids promote root growth in Arabidopsis. Plant Physiol 133(3):1261–1271
Nagase T, Takase H, Sekiya J et al (2015) The axhs1/dwf4 auxin-hypersensitive mutant of Arabidopsis thaliana defines a link for integration of auxin and brassinosteroid mediated root elongation. Plant Biotechnology 32(2):125–137
Nagata T, Saitou K (2009) Regulation of expression of D3-type cyclins and ADP-glucose pyrophosphorylase genes by sugar, cytokinin and ABA in sweet potato (Ipomoea batatas Lam.). Plant Production. Science 12(4):434–442
Nakaya M, Tsukaya H, Murakami N et al (2002) Brassinosteroids control the proliferation of leaf cells of Arabidopsis thaliana. Plant Cell Physiol 43(2):239–244
Nam KH, Li J (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110(2):203–212
Nemhauser J L, Mockler T C, Chory J (2004) Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol 2(9):e258
Ni J, Wang GH, Zhu ZX, Zhang HH, Wu YR, Wu P (2011) OsIAA23-mediated auxin signaling defines postembryonic maintenance of QC in rice. Plant J 68(3):433–442
Okushima Y, Overvoorde PJ, Arima K et al (2005) Functional genomic analysis of the AUXIN RESPONSE FACTOR gene familymembers in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17(2):444–463
Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19(1):118–130
Orman-Ligeza B, Parizot B, Gantet PP et al (2013) Post-embryonic root organogenesis in cereals: branching out from model plants. Trends Plant Sci 18(8):459–467
Pi L, Aichinger E, Vandergraaff E, Llavata-Peris C, Weijers D, Hennig L et al (2015) Organizer-derived WOX5 signal maintains root columella stem cells through chromatin-mediated repression of CDF4 expression. Dev Cell 33(5):576–588
Schlagnhaufer C, Arteca RN, Yopp JH (1984) Evidence that brassinosteroid stimulates auxin-induced ethylene synthesis in mung bean hypocotyls between s-adenosylmethionine and 1-aminocyclopropane – 1- carboxylic acid. Physiol Plant 61(4):555–558
Shimada Y, Goda H, Nakamura A et al (2003) Organ-specific expression of brassinosteroid-biosynthetic genes and distribution of endogenous brassinosteroids in Arabidopsis. Plant Physiol 131(1):287–297
Smolka A, Welander M, Olsson P et al (2009) Involvement of the ARRO-1, gene in adventitious root formation in apple. Plant Sci 177(6):710–715
Sun Y, Fan XY, Cao DM, Tang WQ, He K, Zhu JY, He JX, Bai MY, Zhu SW, Oh E et al (2010) Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell 19(5):765–777
Thomas P, Lee MM, Schiefelbein J (2003) Molecular identification of proline-rich protein genes induced during rootformation in grape (Vitisvinifera L.) stem cuttings. Plant Cell Environ 26(9):1497–1504
Wei Z, Li J (2016) Brassinosteroids regulate root growth, development, and symbiosis. Mol Plant 9(1):86–100
Wells CE, Eissenstat DM (2001) Marked differences in survivorship among apple roots of different diameters. Ecology 82(3):882–892
Yan L, Ma Y, Liu D et al (2012) Structural basis for the impact of phosphorylation on the activation of plant receptor-like kinase BAK1. Cell Res 22(8):1304–1308
Acknowledgements
This work was financially supported by the China Apple Research System (CARS-28) Science and Technology Innovative Engineering Project in the Shaanxi province of China (2015NY114, 2016KTZDNY01-10) and the Yangling Subsidiary Center Project of the National Apple Improvement Center and Collaborative Innovation Center for Shaanxi Fruit Industry Development.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jiangping Mao and Dong Zhang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mao, J., Zhang, D., Li, K. et al. Effect of exogenous Brassinolide (BR) application on the morphology, hormone status, and gene expression of developing lateral roots in Malus hupehensis . Plant Growth Regul 82, 391–401 (2017). https://doi.org/10.1007/s10725-017-0264-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-017-0264-5