Abstract
We examined the effects of 2,4-epibrassinolide (EBR) application on photosynthesis, antioxidant enzyme activity, and Rubisco activase (RCA) gene expression in wheat (Triticum aestivum L.) seedlings under a combination of drought and heat stress. The net photosynthetic rates (Pn) of wheat seedlings decreased significantly, the photosynthetic capability was inhibited, and the activities of superoxide (SOD), peroxidase (POD), catalase (CAT), and RCA as well as the initial and total activity of Rubisco declined under the combined stress. These decreases and inhibitory effects were significantly ameliorated by exogenous EBR application. Three subunits (45–46, 41–42, and 38–39 kDa) of RCA were observed in wheat seedlings. The abundances of the 38–39 kDa and 41–42 kDa subunits were significantly lower in plants subjected to stressful conditions than in unstressed plants. Interestingly, a marked increase in 45–46 kDa RCA was observed under heat or heat combined with drought stress. The abundance of 38–39 kDa RCA in seedlings exposed to heat, drought, or their combination was significantly enhanced by EBR pretreatment, which paralleled the changes in initial Rubisco activity and Pn, but was not consistent with observed mRNA abundance. These results indicated that the larger subunit of RCA (45–46 kDa), which is more thermostable and increased in response to moderate heat stress, and the smaller isoform (38–39 kDa) of RCA may play important roles in maintaining the photosynthetic capability by EBR under stress conditions.
Similar content being viewed by others
Introduction
A broad range of environmental stresses, including cold, heat, drought, and high salinity, are responsible for declines in crop productivity worldwide (Suzuki et al. 2014). Plants are generally subjected to a combination of two or more stresses (Moffat 2002). High temperature and drought are two major environmental factors that limit crop growth and yield, and the combination of these stresses causes many physiological changes that affect crop yield and quality (Rizhsky et al. 2004; Krasensky and Jonak 2012). Climate change models predict that the frequency and intensity of both drought and heat stresses will increase in the near future (Suzuki et al. 2014). Recent studies have revealed that the molecular and metabolic responses of plants to a combination of drought and heat are unique and cannot be directly extrapolated from plant responses to each of these individual stresses (Cairns et al. 2013).
Protein denaturation, enzyme inactivation, reactive oxygen species production, disruption of membrane structure, and damage to ultrastructural cellular components are some of the primary negative effects of drought or heat. In fact, photosynthesis is often the first process that is affected by environmental stresses, such as heat and drought (Pinheiro and Chaves 2011; Suzuki et al. 2014). As the primary limiting factor of net photosynthesis (Salvucci and Crafts-Brandner 2004; Wang et al. 2015), the activation state of Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase) is regulated by Rubisco activase (RCA) via the maintenance of Rubisco catalytic sites in the active state at a high level (Tcherkez 2013). Furthermore, introducing more thermostable RCA into Arabidopsis thaliana L. increases photosynthesis and the growth rate under moderate heat stress (Kurek et al. 2007), which demonstrates that RCA is extremely thermolabile. In addition, the activation state of Rubisco is decreased in plants exposed to drought stress, which consistent with thermal inhibition of RCA activity and limited photosynthesis (Carmo-Silva et al. 2012). Consequently, RCA activity may play an important role in the regulation of photosynthetic capacity under drought or heat stress. Despite extensive studies on drought and heat stress, little is known about the effects of their combination on the expression of Rca.
2,4-Epibrassinolide (EBR) plays prominent roles in various physiological processes, including growth, differentiation, and photosynthesis (Swamy and Rao 2008; Kim et al. 2012). As a potent plant growth regulator, EBR is used to increase the growth and yield of important agricultural crops (Khripach et al. 2000). It can also enhance plant tolerance to a variety of abiotic and biotic stresses (Xia et al. 2009a, b; Sharma et al. 2013; Xi et al. 2013). For example, EBR application ameliorates the stress-induced inhibition of photosynthesis in tomatoes and cucumbers (Ogweno et al. 2008; Yu et al. 2004). RCA is an important factor constraining the photosynthetic potential of plants under heat or drought stress (Crafts-Brandner et al. 2000). However, little is known about the effects of EBR on the activity and expression of RCA in plants subjected to heat or drought stress, and less is known about the effects under the combination of stresses.
Wheat (Triticum aestivum L.) is one of the most important food crops in the world. Increases in temperature and drought incidence associated with global warming pose potential threats to wheat yields worldwide (Liu et al. 2014). In this study, we investigated the effects of EBR pretreatment on photosynthesis, antioxidant enzymes, Rubisco activity, and RCA gene expression under heat or drought stress to explore whether EBR could alleviate stress-induced damage to wheat seedlings.
Materials and methods
Plant materials and treatments
Seeds of the wheat cultivar Yumai 49 were purchased from Henan Agricultural University, Zhengzhou, China. Healthy seeds were first surface sterilized with 0.4 % sodium hypochlorite for 15 min followed by repeated washing with distilled water.
Treated seeds were germinated on glass Petri dishes (15 cm in diameter) with moistened filter paper and allowed to grow. Forty seeds were sown per glass culture dish and kept in dark conditions for the first 3 days. On the fourth day after sowing, seedlings with similar growth in terms of shoot and root lengths were selected and transferred to a 2-L hydroponics culture plastic box containing 1 L of half-strength Hoagland nutrient solution. The boxes were wrapped with black tape to prevent light penetration. Then, the seedlings were cultured in the artificial climate under a 12-h photoperiod, 25 °C/15 °C (day/night) temperature, relative humidity of 75 %, and photosynthetic photon flux density of 400 µmol m−2s−1. During the cultivation period, the nutrition balance was maintained by adding 1 L of nutrient solution to each box at a 2-day interval.
Control seedlings (CK) were maintained at 25 °C and 75 % relative humidity. Drought stress (D) was induced by a 20 % PEG-6000 solution (Sigma–Aldrich Co., St. Louis, MO, USA) for 8 h. Heat stress (H) was carried out by transferring seedlings to a growth chamber pre-conditioned at a temperature of 40 °C for 8 h. The combination stress (DH) was simultaneously imposed by 20 % PEG and a high temperature (40 °C) for 8 h. For the D + EBR, H + EBR, and DH + EBR treatments, seedlings were sprayed with 0.1 mg·L−1 2,4-epibrassinolide once per day for 3 d before they were subjected to stresses. Other seedlings were sprayed with distilled water. At 8 h after treatment, the second fully expanded leaves (i.e., the second from the top) were sampled, frozen immediately in liquid nitrogen, and stored at −80 °C for physiological and gene expression analyses, and photosynthetic parameters were determined.
Measurement of chlorophyll a fluorescence transients, photosynthetic rate, and chlorophyll content
The net photosynthetic rate (Pn) was determined using a portable gas exchange system (LCpro+; ADC, Hoddesdon, UK). Ten plants were measured for each treatment. The chlorophyll (Chl) content of the second blade from top was measured with a Chl meter (SPAD-502; Minolta, Tokyo, Japan). Six plants were measured for every treatment. Three SPAD readings were taken, and mean values were used for the analysis.
The polyphasic rise of the fluorescence transient was measured on the second fully expanded blade from the top after 30 min of dark adaptation using a Plant Efficiency Analyzer (PEA; Hansatech, UK), following the procedures of Strasser and Strasser (1995). The transients were induced by red light of approximately 3000 µmol photons m−2s−1 provided by an array of 6 light-emitting diodes (peak 650 nm) (Zhang et al. 2011). The chlorophyll a fluorescence transients were obtained by 2 s saturating red light and analyzed with the JIP-test (Strasser and Strasser 1995): (a) the fluorescence intensity at 50 µs, considered Fo, when all PSII RCs are open; (b) the maximal fluorescence intensity, Fm, assuming that the excitation intensity is high enough to close all of the RCs of PSII; (c) the fluorescence intensities at 300 µs (K-step) and 2 ms (J-step); (d) WOJ = (Ft − Fo)/(FJ− Fo). ∆WOJ = WOJ (treatment)–WOJ (drought).
Determination of activities of RCA, Rubisco, and antioxidant enzymes
Rubisco activity was measured as described by Jiang et al. (2012). RCA activity was determined using a Rubisco Activase Assay Kit (Genmed Scientifics Inc., Wilmington, DE, USA). Total superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) activity levels were determined as described by Prochazkova et al. (2001).
Total RNA extraction and Rca transcript expression
Total RNA was isolated from wheat leaves with the Spin Column Plant Total RNA Purification Kit (Sangon Biotech, Shanghai, China) according to the manufacturer’s instructions. The cDNA template for real-time RT-PCR was synthesized using the AMV First Strand cDNA Synthesis Kit (Sangon Biotech). Based on sequences in the GenBank database (Accession number: KC776912.1; AF251264.1; AB181991.1), the following gene-specific primers were designed with Primer Premier 5.0 and used for amplification: Rca a 5′-TCTACATCGCTCCTGCTTTCAT-3′ and 5′-AGCTCGCACTGGAATGATTTT-3′; Rca b 5′-GAGGCTGCCGACATTATCAA-3′ and 5′-GTTGTTCACCGTGTACTGCGT-3′; actin, 5′-TCAGAGGAATAAGGGGTACAGG-3′ and 5′-TTTCATACAGCAGGCAAGCA-3′.
Real-time RT-PCR was performed with the ABI StepOne PlusTM Real-Time PCR Detection System (ABI, Waltham, MA, US). Each reaction (20 µL) consisted of 1 µL of diluted cDNA and 10 µL of SybrGreen qPCR Master Mix (ABI). PCR cycling conditions were as follows: denaturation at 95 °C for 3 min and 40 cycles of 95 °C for 10 s and 60 °C for 40 s. Wheat β-actin was used as an internal reference gene to calculate relative transcript levels. Relative gene expression was calculated as described by Livak and Schmittgen (2001).
SDS–PAGE and RCA Western blot analysis
Leaf proteins were extracted in 10 % (w/v) trichloroacetic acid according to Wu and Wang (1984), and protein concentration was determined by Coomassie blue staining. RCA proteins were denatured and separated using a 12.5 % polyacrylamide gel. Protein (30 μg) was added to each well. The resolved proteins were electroblotted to a PVDF membrane (SunBioTech, Beijing, China) and then probed with rabbit anti-RCA antibody (Agrisera, Vännäs, Sweden). The secondary antibody was peroxidase-conjugated goat anti-rabbit IgG. The RCA protein antibody was used at a dilution of 1:6000 and the secondary antibody was used at 1:8000. Expression levels of RCA were estimated by determining band volume with Quantity One (Bio-Rad, Hercules, CA, USA). Band volumes from two replicate plants were averaged.
Statistical analysis
The statistical analyses were conducted using DPS (Data Processing System) (Zhejiang University, China). ANOVA was performed and pairwise differences between treatment means were assessed using Duncan’s multiple range tests at the P < 0.05 probability level.
Results
Effect of EBR pretreatment on wheat seedling photosynthesis under stress
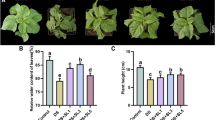
The relative Chl a contents for the D, H, and DH treatments were significantly lower than that of CK seedlings, and was particularly low for the DH treatment (P < 0.05). EBR application retarded the decline in relative Chl a content (Fig. 1a). Similarly, compared with CK, Pn was 26.1, 27.9, and 41.4 % lower for seedlings in the D, H, and DH treatments, respectively (Fig. 1b). These reductions were significantly ameliorated by pretreatment with EBR.
The effect of EBR pretreatment on the relative chlorophyll content (a), net photosynthetic rate (Pn) (b), Fv/Fm (c), and PIabs (d) of wheat seedlings under drought or heat stress. The wheat seedlings were treated as follows: CK distilled water as a control, D 20 % PEG-6000 solution for 8 h, D + EBR 0.1 mg L−1 EBR + 20 % PEG-6000 solution, H 40 °C heat stress, H + EBR 0.1 mg L−1 EBR + 40 °C heat stress, DH 20 % PEG-6000 solution + 40 °C heat stress, DH + EBR 0.1 mg L−1 EBR + 20 % PEG-6000 solution + 40 °C heat stress. Wheat seedlings were pretreated with 0.1 mg L−1 EBR, then exposed to drought or heat stress for 8 h. Data are the means ± SD of three replicates. Different letters indicate significant differences at P < 0.05 according to Duncan’s multiple range tests
Drought and combined drought and heat stress induced significant decreases in Fv/Fm. The decline was markedly alleviated by EBR pretreatment. However, heat stress alone did not induce an obvious decline in Fv/Fm, but, interestingly, a slight reduction was observed in the EBR-pretreated plants under heat conditions (Fig. 1c). Similar to Fv/Fm, photosynthetic performance (PIabs) decreased significantly by drought and the combined stress (Fig. 1d). EBR application remarkably improved the PIabs for seedlings in the D and DH treatments, but resulted in an apparent decline in PIabs for the H treatment. ∆WOJ revealed a K-band with a peak between 0.2 and 0.3 ms (SI Appendix, Fig. S1). In addition, the K-band of plants exposed to stresses increased more rapidly than that of CK plants, especially under combined stress. EBR pretreatment resulted in an apparent decline in ∆WOJ under D and DH conditions, whereas heat stress caused a significant increase in ∆WOJ (SI Appendix, Fig. S1).
Effect of EBR pretreatment on activities of RCA and antioxidant enzymes in wheat seedlings under stress
To further study the mechanism by which EBR regulates CO2 fixation, the total and initial Rubisco activity and RCA activity were determined. Initial and total Rubisco activity, the Rubisco activation state, and RCA activity decreased in response to drought, heat, and, in particular, their combination. However, EBR-pretreated plants had higher Rubisco and RCA activity than plants without EBR treatment (Table 1).
In addition, the activity levels of SOD, POD, and CAT decreased significantly under drought and combined heat and drought stress, especially under the combined stress. However, the decrease was inhibited significantly after EBR pretreatment. Interestingly, their activity levels were markedly increased under heat stress, but reduced after EBR pretreatment (Fig. 2).
Effects of EBR pretreatment on catalase (CAT) (a), peroxidase (POD) (b), and superoxide dismutase (SOD) (c) activity of wheat seedlings under drought or heat stress. The different treatments are described in the legend to Fig. 1. Data are the means ± SD of three replicates. Different letters indicate significant differences at P < 0.05 according to Duncan’s multiple range tests
Effect of EBR pretreatment on the expression of Rca under stress
A qRT-PCR analysis was performed to investigate the effects of stress on Rca expression at the transcript level. As shown in Fig. 3, significant decreases in Rca a transcript level were observed under drought, heat, and combined stress, especially drought stress. The expression of Rca b was significantly higher, by more than 27- and 29-fold, respectively, under heat and combined stress compared with CK, and a slight decrease was observed for the drought treatment. EBR significantly enhanced the expression of both Rca a and Rca b in the drought and combined treatments, but reduced in heat treatment.
qRT-PCR results for Rca a (a) and Rca b (b) of wheat seedlings under drought or heat stress. The different treatments are described in the legend to Fig. 1
Three RCA cross-reacting bands were detected in all treatments. The molecular masses of the observed bands were 45–46 kDa, 41–42 kDa, and 38–39 kDa (Fig. 4). Expression levels of 41–42 kDa and 38–39 kDa RCA were significantly lower in all treatments than in CK plants. Heat and combined stress resulted in a remarkable increase in the 45–46 kDa subunit, but it was significantly decreased under drought stress (Table 2).
Cross-reacting RCA band volumes. Immunoblot analysis of leaf protein extracts of wheat seedlings under drought or heat stress. Proteins were analyzed using SDS-PAGE and immunoblotting. Immunoblots were probed with rabbit anti-Rubisco activase antibody. An equal amount of protein was loaded. The different treatments are described in the legend to Fig. 1
The band volumes of all the three RCA subunits in the D + EBR and DH + EBR treatments were dramatically enhanced compared to those of the D and DH treatments. However, compared with plants under heat stress, a slight decrease in the band volume of the 45–46 kDa and 41–42 kDa subunits, and a dramatic increase in the expression of the 38–39 kDa subunit were observed in the H + EBR treatment (Table 2).
Discussion
Brassinosteroids, a class of plant steroid hormones, play a significant role in the amelioration of various biotic and abiotic stresses by mediating several physiological processes. The brassinosteroid EBR is involved in the regulation of plant development and physiological processes under stress conditions (Yu et al. 2004; Wang et al. 2011; Xu et al. 2015). Previous studies have demonstrated that EBR pretreatment significantly alleviates the inhibition of photosynthesis induced by high temperatures and drought (Ogweno et al. 2008; Hu et al. 2013). Brassinosteroid-induced improvements in photosynthesis might be related to stomatal or non-stomatal factors, or a combination of these (Ali et al. 2008). In the present study, we found that EBR improved photosynthesis and protected the photosynthetic apparatus of wheat under stress; the reductions in Pn, Fv/Fm, and PIabs were significantly retarded by EBR pretreatment. However, there was no obvious change in the intercellular CO2 concentration, implying that EBR improves the efficiency of photosynthetic carbon fixation by increasing the activation state of photosynthetic enzymes and overcoming stomatal limitations (Ali et al. 2008; Ogweno et al. 2008). Similarly, we can also exclude increased antioxidant enzyme activity as the major factor underlying the improved CO2 assimilation in the present study because EBR did not increase antioxidant enzyme activity after heat stress (Fig. 2). However, EBR is involved in the regulation of the Rubisco activation state, which determines photosynthetic performance at high temperatures (Xia et al. 2009a, b). We confirmed this result and showed a significant increase in both initial Rubisco activity and total Rubisco activity in response to EBR (Table 1). The rapid recovery of Calvin cycle enzyme activity minimized the allocation of excess electrons to alternative non-assimilatory pathways, such as the water–water cycle, a process associated with the generation of reactive oxygen species (Miyake and Yokota 2000).
RCA plays an essential role in maintaining Rubisco in an active conformation. In most species, RCA has two subunits, the larger a subunit (43–47 kDa) and the shorter b subunit (41–42 kDa) (Crafts-Brandner et al. 1997). However, three RCA subunits (45–46 kDa, 41–42 kDa, and 38–39 kDa) were found in this study. To our knowledge, the 38–39 kDa subunit was the first subunit reported in wheat plants, although Ristic et al. (2009) also observed three RCA cross reacting bands. It is thought that the wheat genome is composed of three sets of chromosomes, and accordingly has three copies of RCA genes (Ristic et al. 2009) or a complex expression mechanism.
Different RCA subunits may play different roles in photosynthetic heat acclimation. Interactions between RCA subunits ensure a stable RCA structure and maintain initial Rubisco activity under stress conditions (Wang et al. 2010; Chen et al. 2015). Law and Crafts-Brandner (2001) observed a decrease in the abundance of 46-kDa RCA and an increase in 42-kDa RCA after 48 h of heat stress. In contrast, consistent with the findings of Ristic et al. (2009) and Chen et al. (2015), we found that the 45–46 kDa RCA subunit increased significantly after heat or combined with drought stress, suggesting that it plays an important role in photosynthetic acclimation to moderate heat conditions (Chen et al. 2015). A correlation analysis indicated that the small RCA subunit (RCAs) content was highly related to initial Rubisco activity under both heat stress and normal conditions (Wang et al. 2010). Similarly, in our study, the abundance of the 38–39 kDa RCA subunit decreased significantly after all stresses and was dramatically enhanced by EBR application, consistent with the change in initial Rubisco activity. In addition, photosynthetic acclimation was positively correlated with changes in initial Rubisco activity (Wang et al. 2009). However, these changes were not observed for the 45–46 kDa and 41–42 kDa subunits. Therefore, we speculated that EBR increased initial Rubisco activity via enhanced expression of 38–39 kDa RCA, and then further improved the photosynthetic capacity under stress conditions. Changes in Rca transcript abundance in EBR-treated plants were not consistent with the observed changes in initial Rubisco activity and Pn, which suggested that EBR improves the photosynthetic capacity of plants subjected to stress via post-transcriptional regulation.
Furthermore, Immunogold labeling and western blotting have shown that EBR increases the RCA content and this effect can be blocked by inhibitors of redox homeostasis (Jiang et al. 2012). These results strongly suggest that redox homeostasis affects the EBR-induced enhancement in the expression of RCA. Whereas NO contribute to a general plant cell redox homeostasis (Correa-Aragunde er al. 2015). H2O2 and NO are two major signaling molecules during stress responses in plants (Neill et al. 2002). NO plays an important role in the H2O2-dependent induction of plant stress tolerance by EBR (Cui et al. 2011). Additional studies are needed to determine whether NO or H2O2 is involved in the expression of RCA after EBR treatment.
In conclusion, EBR pretreatment enhanced the photosynthetic capacity of wheat leaves subjected to combined heat and drought stress by increasing the 38–39 kDa RCA subunit and initial Rubisco activity.
References
Ali Q, Athar HR, Ashraf M (2008) Modulation of growth, photosynthetic capacity and water relations in salt stressed wheat plants by exogenously applied 24-epibrassinolide. Plant Growth Regul 56:107–116
Cairns JE, Crossa J, Zaidi PH, Grudloyma P, Sanchez C, Araus LJ, thaitad S, Makumbi D, Magorokosho C, Bänziger M, Menkir A, Hearne S, Atlin GN (2013) Identification of drought, heat, and combined drought and heat tolerant donors in Maize. Crop Sci 53:1335–1346
Carmo-Silva AE, Gore MA, Andrade-Sanchez P, French AN, Hunsaker DJ, Salvucci ME (2012) Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ Exp Bot 83:1–11
Chen Y, Wang XM, Zhou L, He Y, Wang D, Qi YH, Jiang DA (2015) Rubisco activase is also a multiple responder to abiotic stresses in rice. Plos One 10: e0140934. doi:10.1371/journal.pone.0140934
Correa-Aragunde N, Foresi N, Lamattina L (2015) Nitric oxide is an ubiquitous signal for maintaining redox balance in plant cells: regulation of ascorbate peroxidase as a case study. J Exp Bot 66:2913–2921
Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. PNAS 97:13430–13435
Crafts-Brandner SJ, van de Loo FJ, Salvucci ME (1997) The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiol 114:439–444
Cui JX, Zhou YH, Ding JG, Xia XJ, Shi K, Chen SC, Asami T, Chen ZX, Yu JQ (2011) Role of nitric oxide in hydrogen peroxide-dependent induction of abiotic stress tolerance by brassinosteroids in cucumber. Plant Cell Environ 34:347–358
Hu WH, Yan XH, Xiao YA, Zeng JJ, Qi HJ, Ogweno JO (2013) 24-Epibrassinosteroid alleviate drought-induced inhibition of photosynthesis in Capsicum annuum. Sci Hortic 150:232–237
Jiang YP, Cheng F, Zhou YH, Xia XJ, Mao WH, Shi K, Chen ZX, Yu JQ (2012) Cellular glutathione redox homeostasis plays an important role in the brassinosteroid-induced increase in CO2 assimilation in Cucumis sativus. New Phytol 194:932–943
Khripach V, Zhabinskii V, de Groot A (2000) Twenty years of brassinosteroids: steroidal plant hormones warrant better crops for the XXI century. Ann Bot 86:441–447
Kim TW, Michniewicz M, Bergmann DC, Wang ZY (2012) Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 482:419–422
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63:1593–1608
Kurek I, Chang TK, Bertain SM, Madrigal A, Liu L, Lassner MW, Zhu GH (2007) Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 19:3230–3241
Law RD, Crafts-Brandner SJ (2001) High temperature stress increases the expression of wheat leaf ribulose-1,5-bisphosphate carboxylase/oxygenase activase protein. Arch Biochem Biophys 386:261–267
Liu B, Liu LL, Tian LY, Cao WX, Zhu Y, Asseng S (2014) Post-heading heat stress and yield impact in winter wheat of China. Glob Chang Biol 20:372–381
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ∆∆C T method. Methods 25:402–408
Miyake C, Yokota A (2000) Determination of the rate of photoreduction of O2 in the water-water cycle in watermelon leaves and enhancement of the rate by limitation of photosynthesis. Plant Cell Physiol 41:335–343
Moffat AS (2002) Finding new ways to protect drought-stricken plants. Science 296:1226–1229
Neill SJ, Desikan R, Clarke A, Hurst R, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signaling molecules in plants. J Exp Bot (53):1237–1247
Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogués S (2008) Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27:49–57
Pinheiro C, Chaves MM (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62:869–882
Prochazkova D, Sairam RK, Srivatava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161:765–771
Ristic Z, Momčilović I, Bukovnik U, Prasad PVV, Fu J, Deridder BP, Elthon TE, Mladenow N (2009) Rubisco activase and wheat productivity under heat-stress conditions. J Exp Bot 60:4003–4014
Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696
Salvucci ME, Crafts-Brandner SJ (2004) Mechanism for deactivation of Rubisco under moderate heat stress. Physiol Plant 122:513–519
Sharma I, Ching E, Saini S, Bhardwaj R, Pati PK (2013) Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol Biochem 69:17–26
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP-test. In: Mathis P (ed) Photosynthesis: from Light to Biosphere, vol V. Kluwer Academic Publishers, Dordrecht, pp 977–980
Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 203:32–43
Swamy KN, Rao SSR (2008) Influence of 28-homobrassinolide on growth, photosynthesis metabolite and essential oil content of geranium[Pelargonium graveolens (L.) Herit]. Am J Plant Physiol 3:173–174
Tcherkez G (2013) Modelling the reaction mechanism of ribulose-1,5-bisphosphate carboxylase/oxygenase and consequences for kinetic parameters. Plant Cell Environ 36:1586–1596
Wang D, Lu Q, Li XF, Jiang QS, Wu JX, Jiang DA (2009) Relationship between Rubisco activase subunits levels and photosynthetic rate in different leaf positions of rice plant. Photosynthetica 47:621–629
Wang GP, Hui Z, Li F, Zhao RM, Zhang J, Wang W (2010) Improvement of heat and drought photosynthetic tolerance in wheat by over accumulation of glycinebetaine. Plant Biotechnol Rep 4:213–222
Wang BL, Zhang JL, Xia XZ, Zhang WH (2011) Ameliorative effect of brassinosteroid and ethylene on germination of cucumber seeds in the presence of sodium chloride. Plant Growth Regul 65:407–413
Wang ZZ, Zheng P, Meng JF, Xi ZM (2015) Effect of exogenous 24-epibrassinolide on chlorophyll fluorescence, leaf surface morphology and cellular ultrastructure of grape seedlings (Vitis vinifera L.) under water stress. Acta Physiol Plant 37:1729–1740
Wu SF, Wang MY (1984) Extraction of proteins for sodium dodecyl sulfate–polyacrylamide gel electrophoresis from protease-rich plant tissues. Anal Biochem 139:100–103
Xi ZM, Wang ZZ, Fang YL, Hu ZY, Hu Y, Deng MM, Zhang ZW (2013) Effects of 24-epibrassinolide on antioxidation defense and osmoregulation systems of young grapevines (V. Vinifera L.) under chilling stress. Plant Growth Regul 71:57–65
Xia JX, Huang LF, Zhou YH, Mao HW, Shi K, Wu JX, Asami T, Chen ZX, Yu JQ (2009a) Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 230:1185–1196
Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen ZX, Yu JQ (2009b) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150:801–814
Xu F, Gao X, Xi ZM, Zhang H, Peng XQ, Wang ZZ, Wang TM (2015) Application of exogenous 24-epibrassinolide enhances proanthocyanidin biosynthesis in Vitis vinifera ‘Cabernet Sauvignon’ berry skin. Plant Growth Regul 75:741–750
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55:1135–1143
Zhang ZH, Jia YJ, Gao HY, Zhang LT, Li HD, Meng QW (2011) Characterization of PSI recovery after chilling-induced photoinhibition in cucumber (Cucumis sativus L.) leaves. Planta 234:883–889
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhao, G., Xu, H., Zhang, P. et al. Effects of 2,4-epibrassinolide on photosynthesis and Rubisco activase gene expression in Triticum aestivum L. seedlings under a combination of drought and heat stress. Plant Growth Regul 81, 377–384 (2017). https://doi.org/10.1007/s10725-016-0214-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0214-7