Abstract
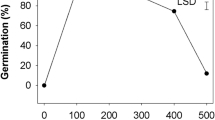
Weedy broomrape species, such as sunflower broomrape (Orobanche cumana Wallr.) and Egyptian broomrape [Phelipanche aegyptiaca Pers. (syn. O. aegyptiaca)], require a period of pre-conditioning before they can respond to germination stimulants. Thus, the sensitivity of weedy broomrape seeds to germination stimulants could be an important factor for broomrape control. In this study, the influence of conditioning agents, conditioning period (0–21 days) and germination stimulants on the germination of sunflower broomrape and Egyptian broomrape seeds was analyzed. Without conditioning, the sunflower and Egyptian broomrape seeds exhibited negligible germination responses to the stimulants. The germination rate of the broomrape seeds increased rapidly with conditioning period and reached a maximum under a conditioning period of 4–10 days; further prolonged conditioning resulted in a decrease in the germination rate. Gibberellic acid (GA3) could not only break the dormancy of the sunflower and Egyptian broomrape seeds but also maintained the high sensitivity of these seeds even after 21 days of conditioning. Furthermore, 100 µM of GA3 induced the germination of the Egyptian broomrape seeds. The stimulants that induced Egyptian broomrape germination were ranked in decreasing order as GR24 (76.8 %), strigol (76.1 %), tobacco root exudates (49.5 %), dehydrocostus lactones (DCL, 39.2 %), and maize root exudates (18 %). In contrast, GA3 did not directly induce sunflower broomrape seed germination, which responded to strigol (62.8 %) > maize root exudates (58.2 %) > GR24 (57.9 %) > tobacco root exudates (41.6 %) > DCL (41.3 %). These results indicate specialized recognition of germination stimulants by sunflower and Egyptian broomrape. This study may contribute to a better understanding of parasitic weed germination and may lead to improved control strategies.




Similar content being viewed by others
References
Awad AA, Sato D, Kusumoto D, Kamioka H, Takeuchi Y, Yoneyama K (2006) Characterization of strigolactones, germination stimulants for the root parasitic plants Striga and Orobanche, produced by maize, millet and sorghum. Plant Growth Regul 48:221–227
Bar Nun N, Plakhine D, Joel DM, Mayer AM (2003) Changes in the activity of the alternative oxidase in Orobanche seeds during conditioning and their possible physiological function. Phytochemistry 64:235–241. doi:10.1016/S0031-9422(03)00165-1
Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH (2003) Secondary metabolite signalling in host-parasitic plant interactions. Curr Opin Plant Biol 6:00065–00067. doi:10.1016/S1369-5266(03)
Chae SH, Yoneyama K, Takeuchi Y, Joel DM (2004) Fluridone and norflurazon, carotenoid-biosynthesis inhibitors, promote seed conditioning and germination of the holoparasite Orobanche minor. Physiol Plant 120:328–337. doi:10.1111/j.0031-9317.2004.0243.x
Eizenberg H, Plakhine D, Hershenhorn J, Kleifeld Y, Rubin B (2003) Resistance to broomrape (Orobanche spp.) in sunflower (Helianthus annuus L.) is temperature dependent. J Exp Bot 54:1305–1311. doi:10.1093/jxb/erg129
El-Ghamrawy N, Salem S, Neumann K (1990) Nature of root exudates of Vicia faba plants in relation to induction of Orobanche crenata seed germination. Angew Bot 64:215–224
Evidente A, Fernández-Aparicio M, Cimmino A, Rubiales D, Andolfi A, Motta A (2009) Peagol and peagoldione, two new strigolactone-like metabolites isolated from pea root exudates. Tetrahedron Lett 50:6955–6958. doi:10.1016/j.tetlet.2009.09.142
Fischer NH, Weidenhamer JD, Bradow JM (1989) Dihydroparthenolide and other sesquiterpene lactones stimulate witchweed germination. Phytochemistry 28:2315–2317. doi:10.1016/S0031-9422(00)97974-3
Gibot-Leclerc S, Corbineau F, Sallé G, Côme D (2004) Responsiveness of Orobanche ramosa L. Seeds to GR 24 as related to temperature, oxygen availability and water potential during preconditioning and subsequent germination. Plant Growth Regul 43:63–71. doi:10.1023/B:GROW.0000038242.77309.73
Goldwasser Y, Kleifeld Y, Plakhine D, Rubin B (1997) Variation in vetch (Vicia spp.) response to Orobanche aegyptiaca. Weed Sci 45:756–762
Joel DM (2000) The long-term approach to parasitic weeds control: manipulation of specific developmental mechanisms of the parasite. Crop Protect 19:753–758. doi:10.1016/S0261-2194(00)00100-9
Joel DM, Chaudhuri SK, Plakhine D, Ziadna H, Steffens JC (2011) Dehydrocostus lactone is exuded from sunflower roots and stimulates germination of the root parasite Orobanche cumana. Phytochemistry 72:624–634. doi:10.1016/j.phytochem.2011.01.037
Kebreab E, Murdoch AJ (1999) A quantitative model for loss of primary dormancy and induction of secondary dormancy in imbibed seeds of Orobanche spp. J Exp Bot 50:211–219. doi:10.1093/jxb/50.331.211
Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15:281–307. doi:10.1079/SSR2005218
Lechat MM, Pouvreau JB, Péron T, Gauthier M, Montiel G, Veronesi C, Todoroki Y, Le Bizec B, Monteau F, Macherel D, Simier P, Thoiron S, Delavault P (2012) PrCYP707A1, an ABA catabolic gene, is a key component of Phelipanche ramosa seed germination in response to the strigolactone analogue GR24. J Exp Bot 63:5311–5322. doi:10.1093/jxb/ers189
Lechat MM, Brun G, Montiel G, Véronési C, Simier P, Thoiron S, Pouvreau JB, Delavault P (2015) Seed response to strigolactone is controlled by abscisic acid-independent DNA methylation in the obligate root parasitic plant, Phelipanche ramosa L. Pomel. J Exp Bot 66:3129–3140. doi:10.1093/jxb/erv119
Lins RD, Colquhoun JB, Cole CM, Mallory-Smith CA (2009) Postemergence small broomrape (Orobanche minor) control in red clover. Weed Technol 19:411–415
Matusova R, Mourik TV, Bouwmeester HJ (2004) Changes in the sensitivity of parasitic weed seeds to germination stimulants. Seed Sci Res 14:335–344
Moral J, Lozano-Baena MD, Rubiales D (2015) Temperature and water stress during conditioning and incubation phase affecting Orobanche crenata seed germination and radicle growth. Front Plant Sci 6:408. doi:10.3389/fpls.2015.00408
Nun NB, Mayer AM (1993) Preconditioning and germination of Orobanche seeds: respiration and protein synthesis. Phytochemistry 34:39–45. doi:10.1016/S0031-9422(00)90779-9
Parker C (2009) Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag Sci 65:453–459. doi:10.1002/ps.1713
Parker C, Riches CR (1993) Orobanche species: the broomrapes. In: Parasitic weeds of the world: biology and control. CAB International, Wallingford, pp 111–164
Parker C, Hitchcock AM, Ramaiah KV (1977) The germination of striga species by crop root exudates; techniques for selecting resistant crop cultivars. In: Proceedings of the Asian-Pacific Weed Science Society 6th conference. Weed Science Society, Asian-Pacific, Jakarta, pp 67–74
Plakhine D, Ziadna H, Joel DM (2009) Is seed conditioning essential for Orobanche germination? Pest Manag Sci 65:492–496. doi:10.1002/ps.1716
Song WJ, Zhou WJ, Jin ZL, Cao DD, Joel DM, Takeuchi Y, Yoneyama K (2005) Germination response of Orobanche seeds subjected to conditioning temperature, water potential and growth regulator treatments. Weed Res 45:467–476. doi:10.1111/j.1365-3180.2005.00477.x
Song WJ, Zhou WJ, Jin ZL, Zhang D, Yoneyama K, Takeuchi Y, Joel DM (2006) Growth regulators restore germination of Orobanche seeds that are conditioned under water stress and suboptimal temperature. Aust J Agric Res 57:1195–1201. doi:10.1071/AR06131
Sukno S, Fernández-Martínez JM, Melero-Vara JM (2001) Temperature effects on the disease reactions of sunflower to infection by Orobanche cumana. Plant Dis 85:553–556. doi:10.1094/PDIS.2001.85.5.553
Takeuchi Y, Omigawa Y, Ogasawara M, Yoneyama K, Konnai M, Worsham AD (1995) Effects of brassinosteroids on conditioning and germination of clover broomrape (Orobanche minor) seeds. Plant Growth Regul 16:153–160. doi:10.1007/BF00029536
Tang QY, Zhang CX (2013) Data processing system (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci 20:254–260. doi:10.1111/j.1744-7917.2012.01519.x
van Hezewijk MJ (1994) Germination ecology of Orobanche crenata: implications for cultural control measures. Dissertation. Vrije Universiteit, Amsterdam
Wegmann K (2006) Germination physiology as a target for Orobanche control. In: Workshop parasitic plant management in sustainable agriculture. Instituto de Tecnologia Química e Biológica, Oeiras, pp 23–24
Xie X, Yoneyama K, Yoneyama K (2010) The strigolactone story. Annu Rev Phytopathol 48:93–117. doi:10.1146/annurev-phyto-073009-114453
Yoneyama K, Xie X, Yoneyama K, Takeuchi Y (2009) Strigolactones: structures and biological activities. Pest Manag Sci 65:467–470. doi:10.1002/ps.1726
Yoneyama K, Awad AA, Xie X, Yoneyama K, Takeuchi Y (2010) Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol 51:1095–1103. doi:10.1093/pcp/pcq055
Yoneyama K, Xie X, Kisugi T, Nomura T, Sekimoto H, Yokota T, Yoneyama K (2011) Characterization of strigolactones exuded by asteraceae plants. Plant Growth Regul 65:495–504. doi:10.1007/s10725-011-9620-z
Zehhar N, Ingouff M, Bouya D, Fer A (2002) Possible involvement of gibberellins and ethylene in Orobanche ramosa germination. Weed Res 42:464–469. doi:10.1046/j.1365-3180.2002.00306.x
Acknowledgments
We are thankful for the funding provided by the National Science and Technology Support Program (2011BAD31B05), and we thank American Journal Experts for editing the manuscript for grammar, clarity, and consistency with a specific emphasis on improving the language of the text and thanks were also given to reviewers and editors for their valuable comments for the improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ye, X., Zhang, M., Dong, S. et al. Conditioning duration and agents involved in broomrape seeds responding to germination stimulants. Plant Growth Regul 81, 221–230 (2017). https://doi.org/10.1007/s10725-016-0199-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0199-2