Abstract
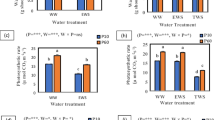
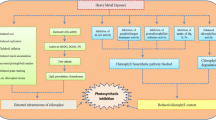
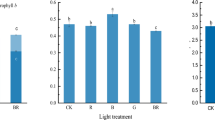
Hydrogen gas (H2) is an endogenous gaseous molecule in plants. Although it is recently described as an important bio-regulator in plants, the role of H2 in high light stress amelioration is largely unknown. This study investigated the mechanism of hydrogen-rich water (HRW)-mediated enhancement of tolerance against high light stress of maize (Zea mays L.) seedlings. Maize seedlings were supplied with different concentrations of HRW and its effects on the plant growth, the chlorophyll fluorescence parameters, the activities of several antioxidative enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR), the levels of superoxide radical (O2 −), hydrogen peroxide (H2O2) and the degrees of the oxidative damage to the membrane lipids under high light stress were examined. With respect to samples treated with high light stress alone, exogenous H2 pretreatments differentially attenuated the damage of photosynthetic apparatus by high irradiance. Further results showed that concentration-dependent effects of HRW were shown in stressed maize seedlings. Exogenous H2 supplement could elevate the activities of antioxidative enzymes, including SOD, CAT, APX, and GR. These results were confirmed by the alleviation of oxidative damage, as indicated by a decrease of the level of O2 − and H2O2 as well as the content of thiobarbituric acid reactive substances. Taken together, our results suggested that exogenous H2 treatment could protect the photosynthetic apparatus from photo-damage through mitigating photo-oxidation enabled by a high level of antioxidative enzyme activities.







Similar content being viewed by others
Abbreviations
- ANOVA:
-
One-way analysis of variance
- APX:
-
Ascorbate peroxidase
- ASC:
-
Ascorbic acid
- CAT:
-
Catalase
- Chl:
-
Chlorophyll
- DAB:
-
3,3′-Diaminobenzidinc
- DI/RC:
-
The specific energy fluxes for dissipation per reaction center
- EDTA:
-
Ethylene diamine tetraacetic acid
- Fo :
-
Initial fluorescence intensity
- Fm :
-
Maximal fluorescence intensity
- Fv :
-
Variable fluorescence in dark-adapted leaves
- Fv/Fm :
-
Maximum quantum yield of PSII photochemistry
- FW:
-
Fresh weight
- GR:
-
Glutathione reductase
- H2 :
-
Hydrogen gas
- H2O2 :
-
Hydrogen peroxide
- HL:
-
High light
- HRW:
-
Hydrogen-rich water
- NADPH:
-
Reduced nicotinamide adenine dinucleotide phosphate
- NBT:
-
Nitro blue tetrazolium
- O2 − :
-
Superoxide radical
- OEC:
-
Oxygen-evolving complex
- PIABS :
-
Performance index on an absorption basis
- Pn :
-
Net Photosynthetic rate
- PPFD:
-
Photosynthetic photon flux density
- PSII:
-
Photosystem II
- RC:
-
Reaction center
- RC/CS:
-
The amount of active PSII reaction centers per excited cross section
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thiobarbituric acid reactive substances
- Vj:
-
The relative fluorescence intensity of J-step
- 1−Vj:
-
The efficiency with which a trapped exciton can move an electron into the electron transport chain further than Q −A
- Vk:
-
The variable fluorescence at 300 μs
References
Adams WW III, Demmig-Adams B (2004) Chlorophyll fluorescence as a tool to monitor plant response to the environment. In: Papageogiou G, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 583–604
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Appenroth KJ, Stockel J, Srivastava A, Strasser RJ (2001) Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ Pollut 115:49–64
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Asada K, Kiso K, Yoshikawa K (1974) Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J Biol Chem 249:2175–2181
Azra Y, Basra SMA, Muhammad F, u Hafeez R, Nazim H, u Habib RA (2013) Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regul 69:225–233
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Baker NR, Horton P (1987) Chlorophyll fluorescence quenching during photoinhibition. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) photoinhibition. Elsevier Science Publishers, Amsterdam, pp 145–168
Barbara DA, William WA III (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172(1):11–21
Boichenko EA (1947) Hydrogenase from isolated chloroplasts. Biokhimiya 12:153–162
Bowler C, Fluhr R (2000) The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci 5:242–246
Bowler C, Montagu MV, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Burritt DJ, Mackenzie S (2003) Antioxidant metabolism during acclimation of Begonia × erythrophylla to high light levels. Ann Bot (London) 91:783–794
Cardinal JS, Zhan J, Wang Y, Sugimoto R, Tsung A, McCurry KR, Billiar TR, Nakao A (2010) Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int 77:101–109
Cavender-Bares J, Bazzaz FA (2004) From leaves to ecosystems: using chlorophyll fluorescence to access photosynthesis and plant function in ecological studies. In: Papageogiou G, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 737–755
Chen WP, Li PH (2001) Chilling-induced Ca2+ overload enhances production of active oxygen species in maize (Zea mays L.) cultured cells: the effect of abscisic acid treatment. Plant Cell Environ 24:791–800
Chen HX, Li WJ, An SZ, Gao HY (2004) Dissipation of excess energy in Mehler-peroxidase reaction in Rumex leaves during salt shock. Photosynthetica 42(1):117–122
Chen CH, Manaenko A, Zhan Y, Liu WW, Ostrowki RP, Tang J, Zhang JH (2010) Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience 169:402–414
Chen M, Cui WT, Zhu KK, Xie YJ, Zhang CH, Shen WB (2013) Hydrogen-rich water alleviates aluminum-induced inhibition of root elongation in alfalfa via decreasing nitric oxide production. J Hazard Mat 267:40–47
Christopher K, Dimitrios R (2012) A review on energy comparison of hydrogen production methods from renewable energy sources. Energy Environ Sci 5:6640–6651
Clark AJ, Landolt W, Bucher JB, Strasser RJ (2000) Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ Pollut 109:501–507
Cui WT, Gao CY, Fang P, Lin GQ, Shen WB (2013) Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J Hazard Mat 260:715–724
Demmig B, Bjorkman O (1987) Comparison of the effect of excessive light on chlorophyll fluorescence (77 K) and photon yield of O2 evolution in leaves of higher plants. Planta 171:171–184
Dong Z, Wu L, Kettlewell B, Caldwell CD, Layzell DB (2003) Hydrogen fertilization of soils–is this a benefit of legumes in rotation. Plant Cell Environ 26:1875–1879
Elstner EF, Heupel A (1976) Formation of H2O2 by isolated cell walls from horseradish (Armoracia lapathifolia). Planta 130:175–180
Eva-Mari A, Ivar V, Bertil A (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochem Biophys Acta 1143:113–134
Giannopolitis CN, Ries SK (1977) Superoxide dismutase. I Occurrence in higher plants. Plant Physiol 59:309–314
Golding AL, Dong Z (2010) Hydrogen production by nitrogenase as a potential crop rotation benefit. Environ Chem Lett 8:101–121
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 22:131–160
Han Y, Zhang J, Chen XY, Gao ZZ, Xuan W, Xu S (2008) Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol 177:155–166
Hideg E, Kalai T, Hideg K, Vass I (1998) Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. Biochemistry 237:11405–11411
Huang CS, Kawamura T, Toyoda Y, Nakao A (2010) Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res 44:971–982
Jin QJ, Zhu KK, Cui W, Xie YJ, Han B, Shen WB (2012) Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ 36:956–969
Jin QJ, Zhu KK, Xie YJ, Shen WB (2013) Heme oxygenase-1 is involved in ascorbic acid-induced alleviation of cadmium toxicity in root tissues of Medicago sativa. Plant Soil 366:605–616
Joshua OO, Hu WH, Song XS, Shi K, Mao WH, Zhou YH, Yu JQ (2010) Photoinhibition-induced reduction in photosynthesis is alleviated by abscisic acid, cytokinin and brassinosteroid in detached tomato leaves. Plant Growth Regul 60:175–182
Kajiyama S, Hasegawa G, Asano M, Hosoda H, Fukui M, Nakamura N (2008) Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res 28:137–143
Kruger GHJ, Tsimilli-Michael M, Strasser RJ (1997) Light stress provokes plastic and elastic modifications in structure and function of photosystem II in camellia leaves. Physiol Plant 101:265–277
Liu S, Liu K, Sun Q, Liu W, Xu W, Denoble P, Tao H, Sun X (2011) Consumption of hydrogen water reduces paraquat-induced acute lung injury in rats. J Biomed Biotechnol 30:50–86
Lu CM, Zhang JH (1999) Heat-induced Multiple effects on PSII in wheat Plants. J Plant Physiol 156:259–265
Lubitz W, Reijerse EJ, Messinger J (2008) Solar water-splitting into H2 and O2: design principles of photosystem II and hydrogenases. Energy Environ Sci 1:15–31
Martin RE, Thomas DJ, Tucker DE, Herbert SK (1997) The effects of photoxidative stress on photosystem I measured in vivo in Chlamydomonas. Plant Cell Environ 20:1451–1461
Mehler AH (1951) Studies on reactivities of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys 33:65–77
Michael M, Ilektra S, Theodora K, Chrysovalantou-Irene A, Ioannis T (2011) Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul 65:315–325
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nakashima-Kamimura N, Mori T, Ohsawa I, Asoh S, Ohta S (2009) Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother Pharmacol 64:753–761
Nakos G, Mortenson L (1971) Purification and properties of hydrogenase, an iron sulfur protein, from Clostridium pasteurianum W5. Biochem Biophys Acta 227:576–583
Nath K, Das D (2003) Hydrogen from biomass. Curr Sci 85:265–271
Nevzat E, Okkes A (2014) Nitric oxide improves chilling tolerance of maize by affecting apoplastic antioxidative enzymes in leaves. Plant Growth Regul 72:29–38
Norio M, Shunichi T, Yoshitaka N, Suleyman IA (2007) Photoinhibition of photosystem II under environmental stress. Biochem Biophys Acta 1767:414–421
Oharazawa H, Igarashi T, Yokota T, Fujii H, Suzuki H, Machide M, Takahashi H, Ohta S, Ohsawa I (2010) Protection of the retina by rapid diffusion of hydrogen: administration of hydrogen-loaded eye drops in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci 51:487–492
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S (2007) Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 13:688–694
Oquist G, Chow WS, Anderson JM (1992) Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of photosystem II. Planta 186:450–460
Prasanna M, Suleyman IA, Norio M (2007) Application of low temperature during photoinhibition allows characterization of individual steps in photodamage and repair if photosystem II. Photosynth Res 94:217–224
Renwick GM, Giumarro C, Siegel SM (1964) Hydrogen metabolism in higher plants. Plant Physiol 39:303–306
Sanadze GA (1961) Absorption of molecular hydrogen by green leaves in light. Fiziol Rast 8:555–559
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Schreber U, Bilger W, Neubauer C (1995) Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze E-D, Cladwell MM (eds) Ecophysiology of Photosynthesis. Springer, Germany, pp 49–70
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26:2027–2038
Shirahata S, Hamasaki T, Teruya K (2012) Advanced research on the health benefit of reduced water. Trends Food Sci Tech 23:124–131
Srivastava AG, Strasser RJ (1999) Greening of peas: parallel measurements on 77 k emission spectra, OJIP chlorophyll a fluorescence transient, period four oscillation of the initial fluorescence level, delayed light emission, and P700. Photosynthetica 37(3):365–392
Srivastava A, Juttner F, Strasser RJ (1998) Action of the allelochemical, fischerellin A, on photosystem II. Biochem Biophys Acta 1364:326–336
Stephenson M, Stickland LH (1931) Hydrogenase: a bacterial enzyme activating molecular hydrogen: the properties of the enzyme. Biochem J 25:205–214
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP-test. In: Mathis P (ed) Photosynthesis: from light to biosphere. Kluwer Academic Publishers, Netherlands, pp 977–980
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U and Mohanty P (eds). Probing Photosynthesis: mechanism, regulation and adaptation. Taylor & Francis, UK, pp 445–483
Strasser RJ, Srivastava A, Tsmilli-Michael M (2004) Analysis of the chlorophyll fluorescence transient. In: Papageorgiou G and Govindjee KS (eds). Chlorophyll fluorescence a signature of photosynthesis. Kluwer Academic Publishers, Netherlands, pp 321–362
Sun Q, Kang ZM, Cai JM, Liu WW, Liu Y, Zhang JH, Denoble PJ, Tao HY, Sun XJ (2009) Hydrogen-rich saline protects myocardium against ischemia/reperfusion injury in rats. Exp Biol Med (Maywood) 234:1212–1219
Telfer A, Bishop SM, Phillips D, Barber J (1994) Isolated photosynthetic reaction center of photosystem II as a sensitizer for the formation of singlet oxygen. J Biol Chem 269:13244–13253
Thordal CH, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Van Heerden PDR, Tsimilli-Michael M, Kruger GHJ, Strasser RJ (2003) Dark chilling effects on soybean genotypes during vegetative development: parallel studies of CO2 assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation. Physiol Plant 117:476–491
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid rain treated bean plants: protective role of exogenous polyammines. Plant Sci 151:59–66
Wang YX, Zhang HL, Hou PF, Su XY, Zhao PF, Zhao HJ, Liu SC (2014) Foliar-applied salicylic acid alleviates heat and high light stress induced photoinhibition in wheat (Triticum aestivum) during the grain filling stage by modulating the psbA gene transcription and antioxidant defense. Plant Growth Regul 73:289–297
Xie YJ, Mao Y, Lai DW, Zhang W, Shen WB (2012) H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS One 7:e49800
Xu S, Zhu SS, Jiang YL, Wang N, Wang R, Shen WB, Yang J (2013) Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant Soil 370:47–57
Yamamoto Y, Aminaka R, Yoshioka M, Khatoon M, Komayama K, Takenaka D, Yamashita A, Nijo N, Inagawa K, Morita N, Sasaki T (2008) Quality control of photosystem II: impact of light and heat stresses. Photosynth Res 98:589–608
Yamauchi Y, Sugimoto Y (2010) Effect of protein modification by malondialdehyde on the interaction between the oxygen-evolving complex 33 kDa protein and photosystem II core proteins. Planta 231:1077–1088
Ye L, Gao HY, Zou Q (2000) Responses of the antioxidant systems and xanthophyll cycle in phaseolus vulgaris to the combined stress of high irradiance and high temperature. Photosynthetica 38(2):205–210
Zeng JQ, Zhang MY, Sun XJ (2013) Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS One 8:e71038
Zheng XF, Mao YF, Cai JM, Li YH, Liu WW, Sun PL, Zhang JH, Sun XJ, Yuan HB (2009) Hydrogen-rich saline protects against intestinal ischemia/reperfusion injury in rats. Free Radic Res 43:478–484
Zheng XF, Sun XJ, Xia ZF (2011) Hydrogen resuscitation, a new cytoprotective approach. Clin Exp Pharmacol Physiol 38:155–163
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Zhao, X., Wang, Z. et al. Protective effects of hydrogen-rich water on the photosynthetic apparatus of maize seedlings (Zea mays L.) as a result of an increase in antioxidant enzyme activities under high light stress. Plant Growth Regul 77, 43–56 (2015). https://doi.org/10.1007/s10725-015-0033-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0033-2